Novel germplasm of tepary and other Phaseolus bean wild relatives from dry areas of southwestern USA
Daniel G. Deboucka,*, Richard C. Prattb, Sarah Dohlec, Timothy Porchd, Marcela Santaellaa, Luis Guillermo Santosa and Milan O. Urbane
aGenetic Resources Program, International Center for Tropical Agriculture (CIAT), Palmira, Colombia
bNew Mexico State University, Department of Plant and Environmental Sciences, Las Cruces, New Mexico, USA
cUnited States Department of Agriculture, Agriculture Research Service (USDA-ARS), Pullman, Washington, USA
dUnited States Department of Agriculture, Agriculture Research Service (USDA-ARS), Mayagüez, Puerto Rico
eBean Program, International Center for Tropical Agriculture (CIAT), Palmira, Colombia now at International Center for Biosaline Agriculture (ICBA), Dubai, United Arab Emirates
*Corresponding author: Daniel Debouck (d.debouck@cgiar.org)
Abstract: Heat and drought stresses threaten global bean production. Additional genetic resources are needed in genebanks for future improvement of bean crops through breeding for tolerance. The USA southwestern Sky Island mountains contain such genetic resources that have not been adequately collected nor characterized. Continuing the work done in 2023 (during which 14 populations were identified and described), a 9-day exploration in 2024 in southern New Mexico and Arizona for wild teparies and other Phaseolus species resulted in the collection of herbarium and seed samples of 18 populations of P. acutifolius, one each of P. angustissimus and P. filiformis, two of P. grayanus, three of P. maculatus and possibly three of P. montanus, or 28 populations in total. Samples of nodules and soil of rhizosphere were also collected. Outcomes and ways to improve these exploration endeavours are discussed.
Keywords: Crop wild relatives, drought stress, germplasm exploration, high-temperature stress, tepary, Phaseolus acutifolius
Introduction
The megadrought of western North America with effects extending south to the Central American Dry Corridor is a current and historic climatic feature (Williams et al, 2020; McKinnon et al, 2021; IPCC, 2023; Chen et al, 2025). That water-limited area is a huge arc extending in the north from Saskatchewan (Canada) to North Dakota and Nebraska south to New Mexico (USA), Chihuahua and Zacatecas (Mexico) and ending in eastern Guatemala. The present combination of record high temperatures, prolonged drought and limited water resources can have profound implications for agricultural systems. Increasingly, growing urban areas and agriculture will compete for ever scarcer fresh-water resources. Farmers in remote areas will likely seek grains with sufficient value (e.g. barley for breweries, beans for export to Mexico and Central America, quinoa for specialty markets) to cover production and postharvest costs such as transportation. High-value grains capable of providing more stable income with a lower water requirement will be essential. Crops and cultivars highly resistant to drought and heat stresses are now high on the agenda of agronomists and breeders in that area (Parker et al, 2023; Silber-Coats et al, 2025), but also in other arid regions of the world e.g. Africa (Assefa et al, 2019).
While the vast American arc aforementioned currently has many introduced crops, tepary bean (Phaseolus acutifolius Asa Gray) has long been known as a native drought and heat tolerant crop (Freeman, 1912) as it was grown by the pre-Columbian peoples of the Southwest (Carter, 1945; Kaplan, 1956); seeds have been dated by accelerator mass spectrometry to at least 2,000 years before present (Kaplan and Lynch, 1999). In addition to drought (Parsons and Howe, 1984; Barrera et al, 2024) and high temperature tolerance (Lin and Markhart III, 1996; Cruz et al, 2023), tepary is resistant to several diseases (ashy stem blight: Miklas et al, 1998; common bacterial blight: Coyne et al, 1963; Bean Golden Mosaic Virus (later shown to be Bean Golden Yellow Mosaic Virus): Miklas and Santiago, 1996; Bean Golden Yellow Mosaic Virus: Porch et al, 2021; Bean Common Mosaic Necrosis Virus: Bornowski et al, 2023; rust: Miklas and Stavely, 1998) and pests (bruchids: Shade et al, 1987; Jiménez et al, 2017; thrip and leafhopper: Porch and Estévez de Jensen, 2024). Further, some accessions of P. acutifolius are tolerant to low temperatures (Souter et al, 2017) and salinity (Bayuelo-Jiménez et al, 2002).
Not surprisingly, there have been many attempts to transfer the useful traits of tepary into the common bean through interspecific hybridization (Pratt and Nabhan, 1988), but with limited success because of the genetic distance between them (Debouck, 1999; Barrera et al, 2022). However, the technological context of bean breeding is changing, given the development of the genome sequences for both species, marker assisted selection, and genomic technologies (Schmutz et al, 2014; Moghaddham et al, 2021; Parker et al, 2023; Wang et al, 2024). Genome editing through the CRISPR-Cas 9 of 2012 and subsequent improvements are also quickly changing the field of potential pathways for cultivar development (Bandyopadhyay et al, 2020; Jha et al, 2022; Singh et al, 2024). There are a lot of possibilities given the high level of synteny between tepary and common bean (Gujaria-Verma et al, 2016; Moghaddham et al, 2021).
Transfer of candidate genes from common bean for highly heritable traits such as seed size, seed colour, growth habit, and disease and pest resistance, may prove more expedient than attempting to transfer complex polygenic traits such as heat and/or drought tolerance from tepary into common bean. Incidentally, the approach to breed tepary itself is increasingly being considered in its traditional areas of cultivation (Pratt et al, 2023) but also in sub-Saharan Africa (Mwale et al, 2020) and in the tropics (Porch et al, 2024). So, it might be faster to breed tepary itself with molecular markers developed using the reference genome sequences of both species, based on the high level of synteny between them.
Further, the food processing industry may offer opportunities for bean seed types outside the current market classes (Voysest and Dessert, 1991) and the traditional ways of cooking. These factors are likely contributing to the increasing trend in P. acutifolius germplasm requests from the United States Department of Agriculture (USDA) genebank (Dohle, 2024).
The success of these new approaches depends heavily on the availability of genetic variability in tepary bean. Unfortunately, a lot of landraces went extinct, first when the Spaniards introduced new irrigation techniques into Mesoamerica in the 16th century onwards, and second in the 1930s onwards when water pumps with fuel-based engines changed the watering systems in the Southwest and other parts of Aridoamerica (Nabhan and Felger, 1978; Pratt et al, 2023). Some cultivated germplasm has been collected from the historic range of tepary cultivation from northern Arizona (Carter, 1945; Nabhan and Felger, 1978) to Guanacaste in Costa Rica (Debouck, 1992), and it is conserved in several genebanks. Once internal duplicates are identified, there are about 100 different landraces of tepary bean in the major germplasm collections for that crop: USDA, the International Center for Tropical Agriculture (CIAT) and INIFAP (Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias of Mexico).
Given this inadequate representation of the intraspecific genetic variation in the genebanks and their obligation to anticipate breeders’ needs instead of reacting to them, it is imperative to expand the reservoir of available genetic diversity. The greatest benefit will come from the two wild forms of tepary (often named var. acutifolius Asa Gray and var. tenuifolius Asa Gray: Delgado-Salinas, 1985). The distribution of these wild forms extends from the southwestern USA (central Arizona, southern New Mexico, and the Trans-Pecos region of Texas) to Michoacán, Mexico (Nabhan and Felger, 1978; Debouck, 2021). While there might be ecological reasons for the recognition of these two varieties (Pratt and Nabhan, 1988), genetic evidence indicates that it is still an open question (Muñoz-Florez et al, 2006; Blair et al, 2012). A sister species and wild relative of tepary, P. montanus Brandegee (synonym P. parvifolius Freytag), strikingly similar in appearance to, but separated from var. tenuifolius based on biochemical (Florez-Ramos et al, 2004) and molecular evidence (Zink and Nagl, 1998a; Muñoz-Florez et al, 2006; Blair et al, 2012), extends from southeastern Arizona down to Jalapa, Guatemala through several regions of the Pacific side of Mexico (Debouck, 2021). Because of its presence in the Chiricahua Mountains (Debouck, 2019), an additional question is whether P. montanus is present in western New Mexico (the Arizona-New Mexico state border line likely not being an ecological barrier, at least up to the continental divide).
Another group of wild beans and with some genetic relationship with common bean is section Rugosi (Zink and Nagl, 1998b; Delgado-Salinas et al, 2006). It includes P. angustissimus Asa Gray, P. carterae Freytag & Debouck and P. filiformis Bentham (Freytag and Debouck, 2002; Dohle et al, 2019). Gene transfer to common bean from these relatives seems very difficult (Maréchal and Baudoin, 1978). Which traits could be of interest for introgression into tepary or into common bean? Wild teparies and these Rugosi species might be tolerant to drought, salinity and freezing temperatures (Bayuelo-Jiménez et al, 2002; Balasubramanian et al, 2004). In conclusion, the main purpose of this project is to increase the representation of wild teparies and species of the section Rugosi from New Mexico, where collection to date has been inadequate, in the USDA collection and later that of CIAT. Further, given the rapid progress in comparative pangenome analysis (e.g. Khan et al, 2024), it might be good foresight to collect other Phaseolus species from New Mexico as the opportunity arises in the field.
At the start of this project in mid-2023 there were two accessions of wild P. acutifolius (PI 640990, a collection by Oliver Wendel Norvell of November 1969 and PI 702622, a collection by Richard Pratt of October 2017; see also Figure 1) and two accessions of P. angustissimus (PI 535272, a collection by Russ Buhrow and PI 535273, a collection by George Frederick Freytag) from New Mexico in the genebanks of USDA-Pullman and CIAT-Palmira. Accordingly, a joint exploration was carried out in fall 2023. Unfortunately, as it often happens in desert areas, 2023 was a special year with below normal, erratic and late rainfalls in the counties of interest in southern New Mexico. However, several populations of wild teparies yielded some seeds from dried plants of the previous year, as did return visits to three sites (namely Big Burro Mountains and two sites in the Organ Mountains) with delayed flowering (Debouck et al, 2023). Those collections have been successfully increased in the glasshouse of the USDA Pullman during the spring of 2024 (S. Dohle, personal communication, 2024). Finally, the finding of a new species of rhizobium in nodules of P. filiformis tolerant to salinity and high temperatures in Baja California (Rocha et al, 2020) justifies the continued sampling (initiated in 2023) of rhizosphere microorganisms in wild teparies and Rugosi species to capture effective nitrogen fixation under these abiotic stresses.
Materials and methods
Populations of target species
The team used two primary kinds of information to decide where to sample: the study of herbaria (identified by their acronyms: Thiers, 2023) keeping samples of Phaseolus sensu stricto and the sightings posted on iNaturalist (https://www.inaturalist.org/). The former source by personal visits to 86 herbaria collections since 1978 (Debouck, 2021, p. 102-103) and the study of 16 herbaria through the Southwestern Environmental Information Network (SEINet) portal (https://swbiodiversity.org/seinet/collections/list.php/) in April 2023 (Supplemental Material 1) gave a total of 189 populations for the state of New Mexico for six species (P. acutifolius (29 populations), P. angustissimus (67), P. filiformis (9), P. grayanus (37), P. maculatus (40) and P. parvulus (7)) covering a period of field collecting between 1849–2014 (Supplemental Table 1). For the 29 populations of wild tepary, the three coordinates were provided by the collector(s) only for three locations (one by global positioning system (GPS)). The second source of information provided GPS coordinates and a colour picture collected by citizens in the year 2024, and some in 2023. In addition, photos and descriptions of habitat preferences of wild teparies were provided to several area hikers. They subsequently reported to us possible sightings during their hikes. These multiple strategies allowed us to find concordance between prospective collection sites and areas where (more) favourable seasonal rainfall patterns had occurred. On the other hand, germplasm of the type localities from original descriptions was also taken into account, given its importance for future taxonomic and genomic studies. For New Mexico, this representation of type materials in genebanks refers to P. acutifolius var. tenuifolius (‘near the copper mines, New Mexico’ in October 1851), P. angustissimus (‘hill-sides above Doña Ana’ in July 1851), P. grayanus (‘San Luís Mountains’ in September 1893) and P. parvulus (‘Pinos Altos Mountains’ in August 1880) (Wooton and Standley, 1915; Freytag and Debouck, 2002).
Timing of collection
In collecting in the northern Chihuahuan Desert and surrounding dry areas (Dick-Peddie, 1993; Cornett, 2013), it was critically important to know how the populations revealed by the two approaches aforementioned had their flowering and pod setting affected by the North American monsoon, in essence variable from year to year (Nolin and Hall-McKim 2006; Reichenbacher and Peachey, 2025). Based on the experience of 2023, monitoring of rain accumulation and distribution was started in July 2024 through late September 2024 for an area covering southwestern New Mexico (roughly south of 33° 15’ latitude North and from 106° 27’ longitude West) extending beyond the state border with Arizona to include Cochise and Greenlee counties. That area was the one containing most populations of target species and the object of the grants. Rainfall information provided by the US Drought Monitor (https://www.droughtmonitor.unl.edu) (Supplemental Figure 1) was complemented with invaluable field visits and consultations with staff of the US Forest Service (FS) and the Bureau of Land Management (BLM). Such field visits indicated that the Peloncillo, the Florida and the southern Black Range Mountains (almost no rain) would not be fruitful in terms of seed production, and to concentrate exploration instead in 2024 in the Gila region (see Figure 1). For example, information provided by a ranger from the Southwestern Research Station (SRS) near Portal, Arizona, on 23 September 2024 that water was still running in Turkey Creek while not in Cave Creek in the Chiricahua Mountains was key for the team to decide to cross the border into Arizona in search of P. montanus (see Discussion).
Implements used during field work were indicated elsewhere (Debouck, 1988; Moss and Guarino, 1995). GPS coordinates were obtained from a Garmin GPSmap 62S receiver and checked against a second GPS receiver (Garmin Etrex 32X) for any significant deviation (that did not happen: see Table 3 in Debouck et al, 2025), while primary elevation readings were provided by a barometric altimeter Thommen 3D-16 (0-5000). The application OnX Backcountry (OnX Maps Inc., Missoula, Montana) installed on the smartphone of one participant gave further validation to these direct readings, as well as valuable data such as offline maps, names of places and landmarks, and property ownership. Trying to get the right geographic coordinates was doubly important: first, to have the possibility of getting back to the same population for any additional sampling in the same or subsequent season, and second, to monitor the fate of these populations over time. Many collections by Edward Lee Greene, Henry Hurd Rusby, Elmer Ottis Wooton and Charles Wright in the 1850s–1900s lack accurate data about location, making our ability to monitor changes since these early observations difficult, if not impossible. Further, ensuring the exact coordinates is critically important in relation to future germplasm evaluation for stresses related to location such as drought, extreme temperatures or soil limitations (salinity, nutrient deficiency or excess in minor elements). For managing the time at the collection sites and to improve sampling, communication was critical, and two pairs of radios (‘walkie-talkies’) were found useful to link members of the team looking for plants in different parts of a single location.
Sampling and data collection
It was a deliberate strategy to sample as many plants as possible at a single site and therefore the team often worked in pairs or individually for half an hour (often longer) in search of additional plants in a population. The reason for this strategy aimed at collecting genetic diversity lies in the cleistogamous reproduction of tepary (Lord and Kohorn, 1986). The sampling of the populations should also be targeted at the conservation of rare alleles. The example of resistance to bruchids in wild common bean (Osborn et al, 1986, 1988) – where plants having the right arcelin causing antibiosis were less than 20% in the original populations – clearly advocates for thorough sampling. The data taken at collection sites follow the guidelines proposed elsewhere (Debouck, 1988; Moss and Guarino, 1995), and were reflected in the labels of the herbarium vouchers (Supplemental Material 2). Vegetation types were described based on the classification of Castetter (1956), Dick-Peddie (1993) and O’Kane (2025). Vernacular names of plants followed Hitchcock (1971a,b) and Dodson’s guide (2012). GPS coordinates were checked against the atlas and gazetteer of Arizona (DeLorme, 2008) and New Mexico (DeLorme, 2012) and web-based topographic maps provided by CalTopo – Backcountry Mapping (https://caltopo.com/) and the US Geological Survey (https://www.usgs.gov/programs/national-geospatial-program/topographic-maps). These sources and the comprehensive reference guide of place names in New Mexico produced by Julyan (1998) enable the checking of place names.
Results
General
In 2024, a total of 28 populations were found, including two disclosed in 2023 (Table 1 and detailed information about each population in Supplemental Material 2) for six species (Phaseolus acutifolius and its two variants, P. angustissimus, P. filiformis, P. grayanus, P. maculatus and P. montanus, the latter in southeastern Arizona) during a 9-day exploration. Two populations of wild tepary disclosed in 2023 (#3387, #3390) were revisited in 2024 (successfully) to collect more seed for germplasm conservation; for all other populations found in 2023, prior scouting visits indicated no plant development due to lack of rains (and verified for populations #3392 and #3396). Seeds were collected for conservation for all populations except #3407 (too early), and herbarium specimens were collected for all except #3420 and #3421 (too late) (see Supplemental Table 2).
Table 1. Populations found in chronological order (those highlighted in grey refer to populations found in 2023 for which seeds were sought and found in 2024).
|
Collection No. |
Species |
Latitude N |
Longitude W |
Elevation (masl) |
Date |
|
3406 |
acutifolius |
1,565 |
3-Oct-2024 |
||
|
3407 |
acutifolius |
32° 18’ 19.0” |
1,740 |
3-Oct-2024 |
|
|
3408 |
acutifolius |
1,859 |
3-Oct-2024 |
||
|
3387 |
acutifolius |
32° 22’ 08.6” |
106° 33’ 34.7” |
1,750 |
4-Oct-2024 |
|
3409 |
acutifolius |
1,893 |
4-Oct-2024 |
||
|
3390 |
acutifolius |
32° 20’ 16.6” |
106° 35’ 10.7” |
1,757 |
5-Oct-2024 |
|
3410 |
acutifolius |
1,639 |
5-Oct-2024 |
||
|
3411 |
filiformis |
32° 17’ 34.0” |
106° 35’ 39.1” |
1,647 |
5-Oct-2024 |
|
acutifolius |
32° 02’ 18.8” |
106° 57’ 23.0” |
1,288 |
6-Oct-2024 |
|
|
3413 |
acutifolius |
31° 53’ 13.6” |
109° 12’ 30.6” |
1,663 |
7-Oct-2024 |
|
3414 |
montanus |
31° 53’ 13.6” |
109° 12’ 30.6” |
1,663 |
7-Oct-2024 |
|
3415 |
grayanus |
31° 54’ 33.2” |
109° 15’ 09.3” |
1,967 |
7-Oct-2024 |
|
3416 |
montanus |
31° 54’ 33.1” |
109° 15’ 09.7” |
1,964 |
7-Oct-2024 |
|
3417 |
montanus |
31° 55’ 44.6” |
109° 13’ 10.4” |
1,693 |
7-Oct-2024 |
|
3418 |
acutifolius |
31° 55’ 44.6” |
109° 13’ 10.4” |
1,693 |
7-Oct-2024 |
|
3419 |
maculatus |
32° 39’ 08.3” |
108° 31’ 56.2” |
1,781 |
8-Oct-2024 |
|
3420 |
acutifolius |
32° 39’ 01.3” |
108° 31’ 56.8” |
1,790 |
8-Oct-2024 |
|
3421 |
acutifolius |
32° 47’ 17.8” |
108° 29’ 38.2” |
1,518 |
8-Oct-2024 |
|
3422 |
acutifolius |
32° 51’ 04.8” |
108° 35’ 26.0” |
1,327 |
8-Oct-2024 |
|
3423 |
acutifolius |
33° 02’ 59.0” |
108° 30’ 03.4” |
1,535 |
9-Oct-2024 |
|
3424 |
angustissimus |
32° 57’ 57.6” |
108° 33’ 48.2” |
1,412 |
9-Oct-2024 |
|
3425 |
maculatus |
32° 53’ 32.7” |
108° 14’ 06.4” |
1,991 |
10-Oct-2024 |
|
3426 |
grayanus |
33° 07’ 1.3” |
108° 11’ 57.5” |
2,248 |
10-Oct-2024 |
|
3427 |
maculatus |
33° 13’ 46.2” |
108° 15’ 52.3” |
1,744 |
10-Oct-2024 |
|
3428 |
acutifolius |
33° 13’ 35.9” |
108° 16’ 11.5” |
1,795 |
10-Oct-2024 |
|
3429 |
acutifolius |
33° 10’ 45.0” |
108° 12’ 19.2” |
1,698 |
10-Oct-2024 |
|
3430 |
acutifolius |
33° 05’ 16.6” |
109° 05’ 21.8” |
1,827 |
11-Oct-2024 |
|
3431 |
acutifolius |
32° 57’ 04.6” |
108° 57’ 35.8” |
1,918 |
11-Oct-2024 |
Figure 1. Map showing the number and location of accessions of wild tepary (Phaseolus acutifolius) in the USDA collection before (red dots) and after (blue dots) the explorations of 2023 and 2024. More information about the collections made east of Las Cruces, New Mexico, USA, can be found in Supplemental Figure 3.
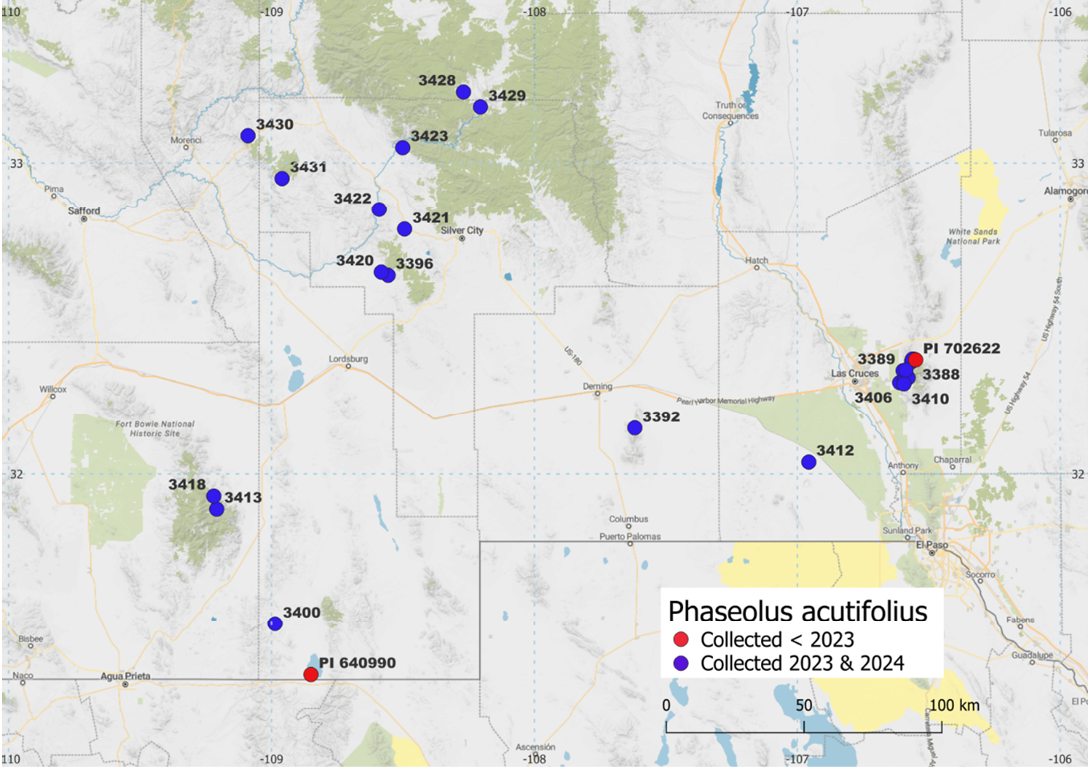
Seeds were taken to the USDA ARS National Plant Germplasm System greenhouses located in Pullman, Washington, for increase, while 96 herbarium specimens were deposited at the New Mexico State University (NMSU) Biology Herbarium (NMC) for conservation and further distribution. Plants from seeds for all the annual species collected during the fall 2024 plant exploration are currently growing in the greenhouses at USDA Pullman (Supplemental Figure 2). Populations may be accessioned and available for distribution likely from 2026 onwards. As can be seen in Figure 1, the collections of 2023 and 2024 resulted in a significant increase in the USDA collection of wild tepary. Samples of nodules or soil around the rhizosphere of the collected plants were obtained for all populations (except two wild tepary populations #3407 and #3409; Supplemental Table 2). Soil and microbe samples are conserved at the New Mexico State University, Las Cruces, New Mexico, USA.
Per species
Phaseolus acutifolius
In 2024, 18 populations were found, most of them at seed dispersal stage. Some populations were noted with relatively broad leaflets (#3387, #3390, #3406, #3407, #3408, #3409, #3410, #3412), while others displayed very narrow ones (#3413, #3418, #3422, #3423, #3428, #3429, #3430, #3431). Two populations (#3420, #3421) were found with all leaves completely dry, making it impossible to prepare good herbarium specimens. In addition to variation in leaf shape, seed colour and size varied (Figure 2). The smallest 100-seed weight was for #3413 at 1.7g, and the largest was for #3408 and #3412, both at 3.5g. One population (#3428) was found on the slope close to the caves in the Gila Cliff Dwellings National Monument. This seems to be the northernmost latitude for P. acutifolius in New Mexico, and apparently, upon evidence available to us, the first record for Catron County. An iNaturalist report indicated the presence of wild tepary in the Aden Lava Flow Wilderness Study Area, Doña Ana County, New Mexico; before reaching the given coordinates, population #3412 (Figure 3 top) was identified and sampled for seed and herbarium specimens. This population is of particular interest because of its location being between the Organ and the Florida Mountains and its low elevation (1,210m barometric). For reasons related to proximity with Las Cruces and distance between transects, the distribution on the western slope of the Organ Mountains (Supplemental Figure 3) is better sampled as compared to other areas (Figure 1). Figure 1 also shows the positive return of scouting in August–September, in line with moisture level predicted in the Gila region by the US Drought Monitor (Supplemental Figure 1). Wild teparies were found in open, quite diverse habitats, from desert treeless grasslands (Figure 4 top) to pine juniper woodland (Figure 4 bottom). They were found in chaparral-like habitat (Figure 5 top) or in dry stream beds (Figure 5 bottom). These habitats match with those reported by Allred and Jercinovic (2020) and Alexander (2025), perhaps with the exception of the Ponderosa pine-oak community, because our sampling has not yet been targeted towards higher elevations (not enough rain in the Black Range in 2023 and 2024!).
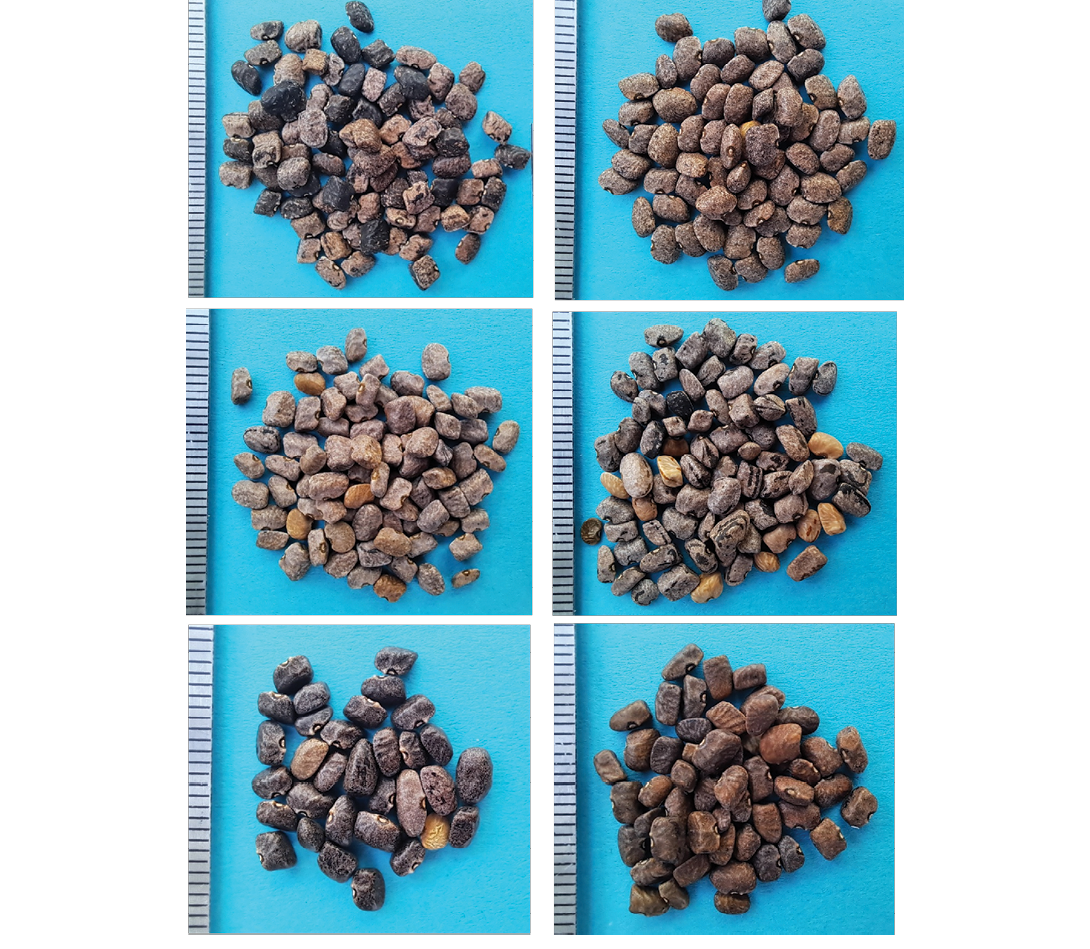

Figure 3. Wild tepary Phaseolus acutifolius Asa Gray. Top: habitat of #3412, Aden Lava Flow Wilderness Area (photo DGD); in absence of cattle grazing, tepary plants (foreground and middle right, arrow) can reach significant development. Lower left: #3430 with narrow leaflets, the lateral ones lobed at base (arrow) (photo SD); lower right: #3406, dry pods as found in most populations at this time; the one to the left with eight seeds (photo SD).

Figure 4. Wild Phaseolus acutifolius habitats. Top: habitat of population #3406, a desert grassland at Sierra Vista Tank in Organ Mountains, Doña Ana County, New Mexico; a few stems can be seen on the Opuntia at foreground (arrow). The rough topography makes the entrance of cattle difficult, while big rocks reduce the effects of drying winds and help collect a bit of air moisture. Bottom: habitat of population #3423, a pine juniper woodland with scattered oaks in Turkey Creek in Brushy Canyon, Grant County, New Mexico (photos DGD).

Figure 5. Wild Phaseolus acutifolius habitats. Top: habitat of population #3387, open low oak chaparral, Aguirre Spring, Organ Mountains, Doña Ana County, New Mexico. Bottom: habitat of population #3396, a dry wash along Red Rock Road in Big Burro Mountains, Grant County, New Mexico. Arrows mark where plants were found in 2023 (photos DGD).
Phaseolus angustissimus
One population (#3424) was found east of Gila, Grant County, New Mexico (there was a previous collection in the area: 4 miles east of Gila, Bear Creek Canyon by Bassett Maguire 11664, 23 May 1935, kept at the herbarium of the New York Botanical Garden (NY) with sprawling stems and very narrow leaflets (Figure 6 lower right); several stems were cut to the base of the plant most likely due to grazing by cattle and/or nibbling by deer. The roadside location (Figure 6 top) perhaps saved this population from being completely wiped out by grazing. While this population was found thanks to an iNaturalist report of September 2024, another population of P. angustissimus similarly reported from a spot inside Silver City was not found. The habitat of population #3424 matches with the one reported by Jason Alexander (2025).

Figure 6. Phaseolus angustissimus Asa Gray, population #3424. Top: habitat, a desert scrubland with few scattered junipers and soil almost half bare; the yellow arrows mark where plants thrive (photo SD). Lower left: close-up of a late purple flower (photo SD). Lower right: a plant with sprawling stems in roadside gully (photo DGD).
Phaseolus filiformis
One small population (#3411; Figure 7) was found (very close to a wild tepary #3410) in the central-southern part of the Organ Mountains, Doña Ana County, New Mexico; because of the location (Cuates Canyon, after an unsuccessful search in Achenbach Canyon, same mountainous range), it might be a new record for the Organ Mountains. It was found at seed dispersal stage (Figure 7 lower right). The population #3393 found in 2023 in Rockhound State Park, Luna County, New Mexico, was visited again for seed in 2024, but because of lack of rains not a single plant was seen (nor for its sympatric wild tepary #3392). The two populations found so far (#3393 and #3411) fall within the diversity of dry and open habitats reported by Allred and Jercinovic (2020) and Alexander (2025).

Figure 7. Phaseolus filiformis Bentham, population #3411. Top: habitat in Cuates Canyon of Organ Mountains, the arrow marking where the plants thrive. Lower left: a late green branch, where all leaflets are quite parallel to sun rays because of very active pulvini, thus difficult to detect. Lower right: an almost dry stem on Opuntia with wrinkled leaflets, twisted pods (arrow) and seeds already dispersed (all photos SD).
Phaseolus grayanus
Two populations (#3415, #3426) were found at green and mature pod stage, often in altitude pine woodland (Figure 8 top). That habitat is one of those reported by Allred and Jercinovic (2020) and Alexander (2025). Many vines were seen without any raceme (Figure 8 lower left), and the low pod productivity may reflect the low amount of rain at these sites in 2024. If flowering is not triggered, more products of photosynthesis will go into the tuberous root (Figure 8 lower right) as the survival strategy of this pluriannual species.

Figure 8. Phaseolus grayanus Wooton & Standley. Top: habitat of population #3415, a pine forest in Cochise County, Arizona (photo DGD). Lower left: if left ungrazed, dense mats of sprawling vines can be seen as in population #3426 in pine woodland, Grant County, New Mexico (photo DGD). Lower right: a 4–5-year-old root (20cm long, diameter 15mm) of plant in population #3426 (photo SD).
Phaseolus maculatus
All three populations (#3419, #3425 and #3427) showed stems with completely dried, tan whitish leaflets, perhaps due to lack of rains in September or scarcity of water in the immediate rocky environment. P. maculatus is normally a pluriannual prostrate legume of the grasslands of the Chihuahuan Desert (Gentry, 1957). Its abundant biomass of palatable shoots explains its extinction in these flatlands due to cattle grazing, while it survives on steep rocky slopes (Figure 9 top). Roadsides (#3419) and pine-oak (#3425) communities were two of the habitats mentioned by Alexander (2025) and one by Allred and Jercinovic (2020). In contrast to wild teparies or P. filiformis (Figure 7 lower right), pods in P. maculatus dehisce less abruptly (Figure 9 lower right). Damage due to seed weevils (Coleoptera Brentidae subfamily Apioninae, J. King, personal communication, 2024) was seen in population #3427.

Figure 9. Phaseolus maculatus Scheele. Top: habitat of population #3427 at Gila Cliff Dwellings, Catron County, New Mexico, a rocky outcrop above a small riverine plain now converted into a parking lot; arrows mark plants with dried sprawling stems (photo DGD). Lower left: population #3419 on a cliff west of Red Rock, Grant County, New Mexico: note the active pulvini putting the leaflets in an upright position (photo SD). Lower right: population #3427 with pods at maturity with 1–3 globose seeds (arrow) (photo SD).
Phaseolus montanus
Three small populations (#3414, #3416, Figure 10, and #3417) were found in 2024, all in Cochise County in southeastern Arizona, while none were identified in New Mexico. As explained below, the team had to enter into Cochise Co., in the Chiricahua Mountains, to verify the presence of the species, and to investigate the species habitat in order to address the question about its presence in New Mexico. The plants were found at flowering and green pod stages, often intermixed with P. acutifolius var. tenuifolius with very narrow leaflets. It was thus important to verify some of the discriminant traits, namely in leaflets (Freytag and Debouck, 2002, page 174) and pods (Brandegee, 1893, page 130), as flowers were not present on all plants (Figure 11). Confirming field observations made in Durango in 1978, and in Guerrero and Jalapa both in 1987 (reported by Freytag and Debouck, 2002), the plants do not exceed 60cm in height and have narrow leaflets (Figure 11) with active pulvini that make their identification challenging in the field.

Figure 10. Phaseolus montanus Brandegee. Top: habitat of population #3414 (shared with P. acutifolius # 3413) (photo DGD). Bottom: leaflets of population #3416 in a shady spot; comparing with Figure 3 lower left, no lobed leaflets are present (photo SD).

Figure 11. Traits used to help identify Phaseolus acutifolius var. tenuifolius (left) and P. montanus (right). Top row: trifoliolate leaves, #3423 (photo SD) and #3414 (photo LGS). Middle two rows: abaxial faces of lateral leaflets; upper: #3413 (photo SD), with arrow marking the formation of an external lobe, and lower: #3414 (photo LGS). Lower row: flowers, #3390 and #3416, the greenish tip of the keel serving as indication of scale (photos DGD). Bottom row: mature pods before shattering: #3428 left (photo SD) and #3417 right (photo SD) (note four developing seeds against eight in P. acutifolius, seven in photograph in upper corner left).
Phaseolus parvulus
One small population (#3395) was found in 2023, in the Pinos Altos Range NE of Silver City, Grant, NM. Given the lack of accuracy in the original species description (‘in the Pinos Altos Mountains, New Mexico’, Greene, 1881, page 217), it could be considered as falling within the range of the type specimen. Together with a few forbs of Compositae and scattered grasses, it thrives on organic soils in the undergrowth of old Ponderosa pine forest (Figure 12); this matches with the habitat mentioned by Allred and Jercinovic (2020) and one of the three habitats indicated by Alexander (2025).

Discussion
These results suggest the following points for discussion, namely on purpose, newness, diversity and prospects. First, with the results of 2023, all six species (P. acutifolius, P. angustissimus, P. filiformis, P. grayanus, P. maculatus and P. parvulus) reported for the state of New Mexico (Wooton and Standley, 1915; Freytag and Debouck, 2002; Allred and Jercinovic, 2020; Alexander, 2025) have been found, and some germplasm has been secured for the USDA genebank. Importantly, the team has learned about vegetation type and microhabitats of each taxon to more readily find additional populations in the future. While the collecting priority was on wild teparies, some germplasm was found for the other species, and this is important for the genebanks serving broader interests in different disciplines (e.g. plant taxonomy, ecophysiology, genomics). In this regard, Arizona has the same six species and in addition, P. montanus and P. ritensis Jones. We concur with Allred and Jercinovic (2020) (page 452) that P. ritensis has not been found yet in New Mexico, and the same for P. montanus.
This observation links with a second point that goes beyond settling a floristic question, given the unique value of P. montanus for reciprocal breeding of common and tepary beans (Barrera et al, 2022). Finding this taxon was debated between the members of the team during preparation, and explained the brief entry into Arizona. We had one record, a collection by Jacob Corwin Blumer #1676 made in 1907 on Paradise slope in the Chiricahua Mountains in the northeastern extreme of the Madrean archipelago (Figure 1 in Van Devender et al, 2013). It was actually a mixed collection of P. acutifolius var. tenuifolius (specimens studied at the herbaria of ARIZ, F, ISC, MO, NMC and NY1) and of P. montanus (specimens studied at the herbaria of CAS, K, L and MIN2) (Debouck, 2019). Before arriving to the Paradise slope not far from the Southwestern Research Station, we found the two species (#3413 and #3414) almost growing side by side. This close proximity repeated itself eastwards from the locality of Paradise (with #3417 as P. montanus and #3418 as P. acutifolius), while population #3416 of P. montanus was found close to P. grayanus #3415. A collection made by Howard Scott Gentry #6472 in Sinaloa, Mexico in 1941 (annotated by one of us in NY; Debouck, 2019) also showed that the two species can be found at the same spot much further south. But the fact that P. montanus is found alone in many places from Guerrero, southern Mexico, south to Jalapa, eastern Guatemala (Freytag and Debouck, 2002; Debouck, 2021) would argue against it being a mere morphological segregant of P. acutifolius var. tenuifolius. Clearly, this close proximity requires further investigation.
A third point relates to sampling diversity of wild teparies in southern New Mexico, where a clearer picture starts to appear thanks to our field work. Extremes in elevation are so far: 1,288m for #3412 and 1,918m for #3431, and extremes in latitude: 31° 30’ 56.8” for #3400 (from 2023 in the Peloncillo Mountains) and 33° 13’ 35.9” for #3428 (from 2024 in the Gila Cliff Dwellings National Monument). The records from herbaria indicated the following range in elevation: the specimen collected by Elmer Ottis Wooton 528 (kept at NY) was found at 1,370m and the specimen collected by J Travis Columbus 1588 (kept at NMCR) was found at 1,980m. So, we have put the limit a bit further towards lower elevation (the collection #3412 of Aden Lava Flow Wilderness). Likewise, the study of herbarium specimens indicated a collection (NMC-15801) by EO Wooton sn in August 1902 ‘Mangas Springs; near Silver City’ as the northernmost location (approxim. 32° 51’), so it seems that we have pushed the limit a bit further north. But the western slope of the Organ Mountains apart (Supplemental Figure 3), our sampling is still unequal and scanty (Figure 1), not because of lack of records – though variously documented (29 wild tepary populations out of 189 of Phaseolus records: Supplemental Table 1), but because of the heavy dependence on intensity and timing of the monsoon rainfall patterns. This uncertainty is common in North American deserts (Beck and Haase, 1969; Larson and Larson, 1997; Nolin and Hall-McKim, 2006; Reichenbacher and Peachey, 2025) and raises the point of how sampling could be improved. One can mention the critical importance of local scouting during August and early September, while the US Drought Monitor gave only a broad picture (Supplemental Figure 1). In this regard, given the lack of meteorological stations in the wilderness of New Mexico, the information provided by rangers of the Bureau of Land Management and of the US Forest Service was extremely valuable, while iNaturalists and informal hikers’ observations gave a 50% chance of accurate data about wild P. acutifolius, the rest being other legumes such as Galactia (for example, for Gallinas canyon NE of San Lorenzo in the Mimbres watershed). The help by iNaturalists can perhaps be made more effective if genebanks put on their websites macrophotos of flowers and pods, cumulating many traits for an accurate identification of target species (Figure 11).
Finally, as noted in the first collection year (2023), grazing in protected areas may be a threat to wild teparies because the soil seed bank might not recover sufficiently to ensure the long-term survival of the populations in the context of the drying Southwest. As a suggestion, from our field work, populations of wild teparies might be selected for the Bureau of Land Management or the US Forest Service to launch a pilot project of in situ conservation, more precisely to address many questions related to the soil seed bank.
Concluding, the field work of 2023 and 2024 resulted in a significant increase of representation of wild tepary germplasm in genebanks, adding 22 new accessions from diverse habitats of the Southwest (New Mexico, Arizona). That ecological diversity may announce an important diversity to be disclosed at the genetic level, thus opening up new prospects for breeding of that crop.
Supplemental Material 1. List of Museums of Natural History and Herbaria where specimens were annotated.
Supplemental Material 2. Detailed information about each population found.
Supplemental Table 1. Numbers of populations by species (verified) and by county of New Mexico.
Supplemental Table 2. Results about the collection of seed, herbarium specimens and nodules/ soil samples of the immediate rhizosphere.
Supplemental Figure 1. Map of New Mexico indicating drought prediction as compared to moisture expected across that state at that date.
Supplemental Figure 2. Photographs of seedlings during the seed increase process at USDA Pullman.
Supplemental Figure 3. Satellite map of the Organ Mountains showing the progress of sampling of wild tepary populations.
Author contributions
This germplasm exploration was originally conceived after a distance workshop during COVID19 lockdown in which participated scientists of the three institutions. For the first (2023) and second (2024) explorations, RP brought the experience and information about suitability of areas from the scouting prior to the field work. He provided many edits and several references about tepary research. SD did the soil sampling in 2024 and collected material of the rhizosphere as well. She added names of several locations of individual collections and provided many high-quality photographs. RP and SD rechecked names of places for all collections reported in Supplemental Material 2. MS and TP participated very actively in the seed collection in 2023 and 2024, respectively. TP provided several insights about tepary breeding and genomics and several references about tepary research. LGS prepared the herbarium specimens in 2023; in 2024 he provided the data about the second GPS recording, photographs and the handling of herbarium specimens. MOU did the soil sampling in 2023 and contributed the rhizosphere samples as well. DGD participated into the identification of populations and collection of data. He did the literature review, wrote the initial draft of the paper and integrated all edits by co-authors. All authors, who were in the field in 2023 and 2024, contributed to the population sampling, read, revised and approved the manuscript.
Acknowledgements
The field work in 2023 was possible thanks to an USDA/ARS National Plant Germplasm System Plant Exploration Office funding for plant exploration in New Mexico for Phaseolus spp. And the field work in Arizona and New Mexico in 2024 was possible thanks to the project ‘Plant Exploration in New Mexico to Collect Wild Species of Phaseolus acutifolius and Phaseolus filiformis Germplasm for Crop Improvement’ sponsored by the USDA/ARS award no. 58-2090-4-042. The authors extend special thanks to Dr Anne Frances and the reviewers for considering the proposal, and the Phaseolus Crop Germplasm Committee for their letter of support. Additional funding was provided by the Plant and Environmental Sciences Department of New Mexico State University, the Genetic Resources Program of CIAT, and the Agriculture Research Service of USDA. Permits to collect herbarium specimens, seed for germplasm conservation and samples of microorganisms and soil were kindly and swiftly granted by the Forest Service of USDA, the Bureau of Land Management of the Department of Interior and the Land Trust of the State of New Mexico. The authors express deep gratitude to Britton Bourland (USDA) for help on the maps.
The help and interest of the following persons: Josh Bachman (NMSU), Stephen Beebe (CIAT), Geoff Bender (SRS), Esteban Bolaños (CIAT), Nury Escobar (CIAT), Sara Fuentes Soriano (NMSU), Lois Grant (NMSU-retired), Gabriela Guerrero Florez (WSU), Anowar Islam (NMSU), Joanie King (NMSU), Juan David Libreros (CIAT), Claudia Maldonado (CIAT), Carla Olson (USDA), Erin Riordan (Arizona-Sonora Desert Museum), Zachary Rogers (NMSU), Kirsten Romig (BLM), Fermin Salas (NPS), John Jairo Sánchez (CIAT), Joe Tohme (CIAT), Eliana Urquijo (CIAT), Carlos Urrea (UNL), Marilyn Warburton (USDA) and Peter Wenzl (CIAT) at different steps of these explorations are deeply acknowledged. The authors express gratitude to a regional director of USDA for interest and to the Editor for helping to improve the manuscript.
Conflict of interest statement
The authors are all interested in increasing knowledge about the native bean species of New Mexico, and adding genetic diversity into USDA and CIAT genebanks, specifically of wild teparies. SD is currently the Curator of the USDA Phaseolus collection and responsible for the bean genebank at the Western Plant Introduction Station of USDA, Pullman, Washington. RP is Professor at New Mexico State University and has investigated the agronomy and ecology of tepary and relatives since the 1980s. TP is a bean breeder and researcher of the Agricultural Research Service based at the Tropical Agricultural Research Station in Mayagüez, Puerto Rico, and has launched tepary breeding since the 2000s. MS is the Manager of the genebank of Future Seeds of the Alliance of Bioversity International and CIAT, based in Palmira, Colombia, and oversees all operations for the seed collections of Phaseolus beans and tropical forages. LGS is Curator of the bean collection kept in the genebank of Future Seeds in Palmira, Colombia. MOU, now at the International Center for Biosaline Agriculture, worked as the bean physiology leader in CIAT, with interest to develop new technologies to measure tolerance to heat, drought, low phosphorus and high aluminium in crop plants. DGD, CIAT Emeritus, has been responsible for CIAT genebank in 1996–2017; now retired he continues writing and sharing information about crops of neotropical origin.
Ethics statement
The primary objective of this collaborative project involving USDA, NMSU and CIAT (the concerned branch of the Alliance of Bioversity International and CIAT) was to increase the genetic diversity in the USDA germplasm collection. Therefore, it was of utmost importance that the different materials were collected in full knowledge of the authorities and with the appropriate permits, and the role of Sarah Dohle, being Staff member of the Agricultural Research Service of USDA, was key in this regard. Where permits were required on public land, they were obtained as follows:
- New Mexico Bureau of Land Management, reference no. 6850 (9300) Laura Hronec, Acting Deputy State Director Division of Lands and Resources (2023 and 2024)
- New Mexico National Forest, inter agency courtesy provided by Jessie Willett, New Mexico Zone Contracting Officer (2023 and 2024)
- New Mexico State Parks, Research Permit #017 (2023) and #027 (2024) provided by Robert Stokes, Program Support Bureau Chief
- Arizona Apache National Forest, inter agency courtesy permission provided by Trace Douglas Timber Management Officer for Apache-Sitgreaves NFs, Alpine and Springerville Ranger Districts (2024)
- Arizona Coronado National Forest, inter agency courtesy permission provided by Douglas Ruppel, District Ranger Douglas Ranger District (2024).
At the Gila Cliff Dwellings National Monument, an in-person permission was provided by Fermin Salas, Superintendent of Gila Cliff Dwellings National Monument, National Park Service, and the Staff of the National Monument very kindly accompanied the collecting team along the official path within the Monument.
References
Alexander, J. A. (2025). Fabaceae – Pea family. In Vascular Plants of New Mexico, ed. K. D. Heil and S. L. O’Kane. Monographs in Systematic Botany, Volume 140. (St. Louis, Missouri, USA: Missouri Botanical Garden Press), 529-598. ISBN: 978-1-935641-30-8.
Allred, K.W., Jercinovic, E.M. (illustr. R. DeWitt Ivey). (2020). Flora Neomexicana III: an illustrated identification manual. Part 2: dicotyledonous plants. 2nd edition. (Durham, North Carolina, USA: Published by the author), 451-455. ISBN: 979-8-651774-81-4.
Assefa, T., Assibi-Mahama, A., Brown, A. V., Cannon, E. K. S., Rubyogo, J. C., Rao, I. M., Blair, M. W., Cannon, S. B. (2019). A review of breeding objectives, genomic resources, and marker-assisted methods in common bean (Phaseolus vulgaris L.). Mol. Breeding 39, 1–23. https://doi.org/10.1007/s11032-018-0920-0.
Balasubramanian, P., Vandenberg, A., Hucl, P., Gusta, L. (2004). Resistance of Phaseolus species to ice crystallization at subzero temperature. Physiol. Plant. 120, 3, 451-457. https://doi.org/10.1111/j.0031-9317.2004.00257.x.
Bandyopadhyay, A., Kancharla, N., Javalkote, V. S., Dasgupta, S., Brutnell, T. P. (2020). CRISPR-Cas12a (Cpf1): a versatile tool in the plant genome editing tool box for agricultural advancement. Front. Plant Sci. 11, 1-17 (584151). https://doi.org/10.3389/fpls.2020.584151.
Barrera, S., Berny Mier y Teran, J. C., Lobaton, J. D., Escobar, R., Gepts, P., Beebe, S., Urrea, C. A. (2022). Large genomic introgression blocks of Phaseolus parvifolius Freytag bean into the common bean enhance the crossability between tepary and common beans. Plant Direct 6 (12): 1-12 (e470). https://doi.org/10.1002/pld3.470.
Barrera, S., Berny Mier y Teran, J.C., Aparicio, J., Diaz, J., León, R., Beebe, S., Urrea, C. A., Gepts, P. (2024). Identification of drought and heat tolerant tepary beans in a multi-environment trial study. Crop Sci. 64, 6, 3399-3416. https://doi.org/10.1002/csc2.21354.
Bayuelo-Jiménez, J., Debouck, D. G., Lynch, J. (2002). Salinity tolerance in Phaseolus species during early vegetative growth. Crop Sci. 42, 6, 2184-2192. https://doi.org/10.2135/cropsci2002.2184.
Beck, W. A., Haase, Y. D. (1969). Historical atlas of New Mexico (Norman, Oklahoma, USA: University of Oklahoma Press), 151p. ISBN 978-0-8061-0817-9.
Blair, M. W., Pantoja, W., Muñoz, L. C. (2012). First use of microsatellite markers in a large collection of cultivated and wild accessions of tepary bean (Phaseolus acutifolius A. Gray). Theor. Appl. Genet. 125, 6, 1137-1147. https://doi.org/10.1007/s00122-012-1900-0.
Bornowski, N., Hart, J. P., Palacios, A. V., Ogg, B., Brick, M. A., Hamilton, J. P., Beaver, J. S., Buell, C. R., Porch, T. (2023). Genetic variation in a tepary bean (Phaseolus acutifolius A. Gray) diversity panel reveals loci associated with biotic stress resistance. The Plant Genome ١٦, e٢٠٣٦٣. https://doi.org/10.1002/tpg2.20363.
Brandegee, T. S. (1893). Flora of the Cape Region of Baja California. Proc. Calif. Acad. Sci. ser. 2, 3, 108-182.
Carter, G. F. (1945). Plant geography and culture history in the American Southwest. Viking Fund Publ. Anthropol. 5, 1-140.
Castetter, E. F. (1956). The vegetation of New Mexico. New Mexico Quaterly 26, 3, 255-288.
Chen, L., Brun, P., Buri, P., Fatichi, S., Gessler, A., McCarthy, M. J., Pellicciotti, F., Stocker, B., Karger, D. N. (2025). Global increase in the occurrence and impact of multiyear droughts. Science 387, 278-284. https://doi.org/10.1126/science.ado4245.
Cornett, J. W. (2013). The Chihuahuan Desert: a brief natural history (Palm Springs, California, USA: Nature Trails Press), 81p. ISBN 978-0-937794-46-3.
Coyne, D. P., Schuster, M. L., Al-Yasiri, S. (1963). Reaction studies of bean species and varieties to common blight and bacterial wilt. Plant Dis. Reptr. 47, 6, 534-537.
Cruz, S., Lobatón, J., Urban, M.O., Ariza-Suarez, D., Raatz, B., Aparicio, J., Mosquera, G., Beebe, S. (2023) Interspecific common bean population derived from Phaseolus acutifolius using a bridging genotype demonstrate useful adaptation to heat tolerance. Front. Plant Sci. 14, 1-15 (1145858). https://doi.org/10.3389/fpls.2023.1145858.
Debouck, D. G. (1988). Phaseolus germplasm exploration. In Genetic resources of Phaseolus beans: their maintenance, domestication, evolution and utilization, ed. P. Gepts (Dordrecht, Holland: Kluwer Academic Publishers), 3-29. ISBN: 90-247-3685-4.
Debouck, D. G. (1992). Frijoles, Phaseolus spp. In Cultivos marginados: otra perspectiva de 1492, ed. E. Hernández Bermejo and J. León (Rome, Italy: Food and Agriculture Organization of the United Nations), 45-60. ISBN: 92-5-303217-0.
Debouck, D. G. (1999). Diversity in Phaseolus species in relation to the common bean. In Common bean improvement in the twentyfirst century, ed. S. P. Singh (Dordrecht, The Netherlands: Kluwer Academic Publishers), 25-52. ISBN: 0-7923-5887-2.
Debouck, D. G. (2019). Cahiers de phaséologie – section Acutifolii Freytag. International Center for Tropical Agriculture, Cali, Colombia. 128p. Available from: https://ciat.cgiar.org/what-we-do/crop-conservation-and-use/ in program files. (accessed on 13 October 2023).
Debouck, D. G. (2021). Phaseolus beans (Leguminosae, Phaseoleae): a checklist and notes on their taxonomy and ecology. J. Bot. Res. Inst. Texas 15, 1, 73-111. https://doi.org/10.17348/jbrit.v15.i1.1052.
Debouck, D. G., Dohle, S., Marquez, D., Pratt, R., Santaella, M., Santos, L. G., Urban, M. (2023). Report on a Phaseolus germplasm exploration in New Mexico, USA, Sep 27 – Oct 8, 2023. New Mexico State University, Las Cruces, New Mexico, United States Department of Agriculture, Pullman, Washington, USA, and International Center for Tropical Agriculture (CIAT), Cali, Colombia. Mimeographed. 26p.
https://cgspace.cgiar.org/items/7767dfac-6d29-4d29-9597-61d8b234db6b.
Debouck, D. G., Dohle, S., Porch, T., Pratt, R., Santos, L. G. (2025). Phaseolus germplasm exploration in New Mexico, USA, Oct 3 – Oct 11, 2024, Report. United States Department of Agriculture, Pullman, Washington, New Mexico State University, Las Cruces, New Mexico, USA, and International Center for Tropical Agriculture (CIAT), Cali, Colombia. Mimeographed. 34p. https://cgspace.cgiar.org/items/6fb6d1aa-b4be-4db1-8885-eea3510872c6.
Delgado-Salinas, A. O. (1985). Systematics of the genus Phaseolus (Leguminosae) in North and Central America. Ph.D. Thesis, Univ. of Texas-Austin, Texas, USA. 363p.
Delgado-Salinas, A., Bibler, R., Lavin, M. (2006). Phylogeny of the genus Phaseolus (Leguminosae): a recent diversification in an ancient landscape. Syst. Bot. 31, 4, 779-791. https://doi.org/10.1600/036364406779695960.
DeLorme. (2008). Arizona Atlas and Gazetteer 7th edition (Yarmouth, Maine, USA: DeLorme), 68p.
DeLorme. (2012). New Mexico Atlas and Gazetteer 6th edition (Yarmouth, Maine, USA: DeLorme), 72p.
Dick-Peddie, W. A. (1993). New Mexico vegetation, past, present, and future (Albuquerque, New Mexico, USA: University of New Mexico Press), 244p. ISBN: 0-8263-2164-X.
Dodson, C. (2012). A guide to plants of the northern Chihuahuan Desert (Albuquerque, New Mexico, USA: The University of New Mexico Press), 194p. ISBN: 978-0-8263-5022-0.
Dohle, S. (2024). USDA National Plant Germplasm System, Phaseolus Crop Germplasm Committee, Curator report presented on August 20, 2024. https://www.ars-grin.gov/documents/cgc/committee/2024%20Phaseolus%20CGC%20Minutes%20Meeting.pdf.
Dohle, S., Berny Mier y Teran, J. C., Egan, A., Kisha, T., Khoury, C. K. (2019). Chapter 4. Wild beans (Phaseolus L.) of North America. In North American Crop Wild Relatives. Volume 2, ed. S. L. Greene, K. A. Williams, C. K. Khoury, M. B. Kantar, L. F. Marek (Berne, Switzerland: Springer Nature AG.), 99-127. https://doi.org/10.1007/978-3-319-97121-6_4.
Florez-Ramos, C. P., Ocampo-Nahar, C. H., Toro-Chica, O. (2004). A biochemical trait helps to recognize Phaseolus parvifolius Freytag in the gene pool of tepary bean. Annu. Rep. Bean Improv. Coop. (USA) 47, 163-164.
Freeman, G. F. (1912). Southwestern beans and teparies. University of Arizona Agricultural Experiment Station. Bulletin 68. Tucson, Arizona, USA. 55p.
Freytag, G. F., Debouck, D. G. (2002). Taxonomy, distribution, and ecology of the genus Phaseolus (Leguminosae-Papilionoideae) in North America, Mexico and Central America. SIDA Bot. Misc. 23, 1-300. https://doi.org/10568/54291.
Gentry, H. S. (1957). Los pastizales de Durango - Estudio ecológico, fisiográfico y florístico (México, D.F., México: Instituto Mexicano de Recursos Naturales Renovables), 361p.
Greene, E. L. (1881). New plants of New Mexico and Arizona. Bot. Gaz. 6, 6, 217-219.
Gujaria-Verma, N., Ramsay, L., Sharpe, A. G., Sanderson, L-A., Debouck, D. G., Tar’an, B., Bett, K. E. (2016). Gene-based SNP discovery in tepary bean (Phaseolus acutifolius) and common bean (P. vulgaris) for diversity analysis and comparative mapping. BMC Genomics 17, 239, 1-16. https://doi.org/10.1186/s12864-016-2499-3.
Hitchcock, A. S. (1950) (1971a). Manual of the grasses of the United States. Revised 2nd edition. USDA Miscel. Public. No. 200. Volume 1 (New York, New York, USA: Dover Publications, Inc.), 1-569. ISBN: 0-486-22717-0.
Hitchcock, A. S. (1971b). Manual of the grasses of the United States. 2nd edition. Volume 2 (New York, New York, USA: Dover Publications, Inc.), 570-1051. ISBN: 0-486-22718-9.
Intergovernmental Panel on Climate Change (IPCC). (2023). Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Core Writing Team, H. Lee and J. Romero (eds). Intergovernmental Panel on Climate Change. Geneva, Switzerland. 184p. https://doi.org/10.59327/IPCC/AR6-9789291691647.
Jha, U. C., Nayyar, H., von Wettberg, E. J. B, Naik, Y. D., Thudi, M., Siddique, K. H. M. (2022). Legume pangenome: status and scope for crop improvement. Plants 11, 1-14 (3041). https://doi.org/10.3390/plants11223041.
Jiménez, J. C., de la Fuente, M., Ordás, B., García Domínguez, L. E., Malvar, R. A. (2017). Resistance categories to Acanthoscelides obtectus (Coleoptera: Bruchidae) in tepary bean (Phaseolus acutifolius), new sources of resistance for dry bean (Phaseolus vulgaris) breeding. Crop Protection 98, 255-266. https://doi.org/10.1016/j.cropro.2017.04.011.
Julyan, R. H. (1998). The place names of New Mexico. Revised edition (Albuquerque, New Mexico, USA: University of New Mexico Press), 385p. ISBN: 0-8263-1689-1.
Kaplan, L. (1956). The cultivated beans of the prehistoric Southwest. Ann. Mo. Bot. Gard. 43, 189-251.
Kaplan, L., Lynch, T. (1999). Phaseolus (Fabaceae) in archaeology: AMS radiocarbon dates and their significance for pre-Colombian agriculture. Econ. Bot. 53, 3, 261-272.
Khan, A. W., Garg, V., Sun, S., Gupta, S., Dudchenko, O., Roorkiwal, M., Chitikineni, A., Bayer, P. E., Shi, C., Upadhyaya, H. D., Bohra, A., Bharadwaj, C., Rouf Mir, R., Baruch, K., Yang, B., Coyne, C. J., Bansal, K. C., Nguyen, H. T., Ronen, G., Lieberman Aiden, E., Veneklaas, E., Siddique, K. H. M., Liu, X., Edwards, D., Varshney, R. K. (2024). Cicer super-pangenome provides insights into species evolution and agronomic trait loci for crop improvement in chickpea. Nature Genetics, 1-20. https://doi.org/10.1038/s41588-024-01760-4.
Larson, P., Larson, L. (1997). The deserts of the Southwest: a Sierra Club naturalist’s guide. Second edition (San Francisco, California, USA: Sierra Club Books), 283p. ISBN: 1-57805-052-9.
Lin, T-Y., Markhart III, A. H. (1996). Phaseolus acutifolius A. Gray is more heat tolerant than P. vulgaris L. in the absence of water stress. Crop Sci. 36, 1, 110-114. https://doi.org/10.2135/cropsci1996.0011183X003600010020x.
Lord, E. M., Kohorn, L. U. (1986). Gynoecial development, pollination, and the path of pollen tube growth in the tepary bean, Phaseolus acutifolius. Amer. J. Bot. 73, 1, 70-78. https://doi.org/10.1002/j.1537-2197.1986.tb09682.x.
Maréchal, R., Baudoin, J.-P. (1978). Observations sur quelques hybrides dans le genre Phaseolus. IV. L’hybride Phaseolus vulgaris x Phaseolus filiformis. Bull. Rech. Agron. Gembloux 13, 3, 233-240.
McKinnon, K. A., Poppick, A., Simpson, I. R. (2021). Hot extremes have become drier in the United States Southwest. Nature Climate Change 11, 7, 598-604. https://doi.org/10.1038/s41558-021-01076-9.
Miklas, P. N., Santiago, J. (1996). Reaction of select tepary bean to Bean Golden Mosaic Virus. HortScience 31, 3, 430-432. https://doi.org/10.21273/HORTSCI.31.3.430.
Miklas, P. N., Schwartz, H. F., Salgado, M. O., Nina, R., Beaver, J. S. (1998). Reaction of select tepary bean to ashy stem blight and Fusarium wilt. HortScience 33, 1, 136-139. https://doi.org/10.21273/HORTSCI.33.1.0136.
Miklas, P. N., Stavely, J. R. (1998). Incomplete dominance of rust resistance in tepary bean. HortScience 33, 1, 143-145. https://doi.org/10.21273/HORTSCI.33.1.0143.
Moghaddam, S. M., Oladzad, A., Koh, C., Ramsay, L., Hart, J. P., Mamidi, S., Hoopes, G., Sreedasyam A., Wiersma, A., Zhao, D., Grimwood, J., Hamilton, J. P., Jenkins, J., Vaillancourt, B., Wood, J. C., Schmutz, J., Kagale, S., Porch, T., Bett, K. E., Buell, C. R., McClean, P. E. (2021). The tepary bean genome provides insight into evolution and domestication under heat stress. Nature Communic. 12, 2638, 1-14. https://doi.org/10.1038/s41467-021-22858-x.
Moss, H., Guarino, L. (1995). Gathering and recording data in the field. In Collecting plant genetic diversity – Technical guidelines, ed. L. Guarino, V. R. Rao and R. Reid (Wallingford, United Kingdom: CAB International), 367-417. ISBN: 0-85198-964-0.
Muñoz-Florez, L. C., Duque, M. C., Debouck, D. G., Blair, M. W. (2006). Taxonomy of tepary bean and wild relatives as determined by amplified fragment length polymorphism (AFLP) markers. Crop Sci. 46, 4, 1744-1754. https://doi.org/10.2135/cropsci2005-12-0475.
Mwale, S. E., Shimelis, H., Mafongoya, P., Mashilo, J. (2020). Breeding tepary bean (Phaseolus acutifolius) for drought adaptation: A review. Plant Breeding 139, 5, 821-833. https://doi.org/10.1111/pbr.12806.
Nabhan, G. P., Felger, R. S. (1978). Teparies in southwestern North America. a biogeographical and ethnohistorical study of Phaseolus acutifolius. Econ. Bot. 32, 1, 3-19.
Nolin, A. W., Hall-McKim, E. A. (2006). Frequency modes of monsoon precipitation in Arizona and New Mexico. Monthly Weather Rev. 134, 12, 3774-3781.
O’Kane, S. L. (2025). Vegetation. In Vascular Plants of New Mexico, ed. K. D. Heil and S.L. O’Kane. Monographs in Systematic Botany, Volume 140 (St. Louis, Missouri, USA: Missouri Botanical Garden Press), 5-22. ISBN: 978-1-935641-30-8.
Osborn, T. C., Blake, T., Gepts, P., Bliss, F. A. (1986). Bean arcelin. 2. Genetic variation, inheritance and linkage relationships of a novel seed protein of Phaseolus vulgaris L. Theor. Appl. Genet. 71, 6, 847-855.
Osborn, T. C., Alexander, D. C., Sun, S. S. M., Cardona, C., Bliss, F. A. (1988). Insecticidal activity and lectin homology of arcelin seed protein. Science 240, 207-210. https://doi.org/10.1126/science.240.4849.207.
Parker, T. A, Acosta-Gallegos, J., Beaver, J., Brick, M., Brown, J. K., Cichy, K., Debouck, D. G., Delgado-Salinas, A., Dohle, S., Ernest, E., Estevez de Jensen, C., Gómez, F., Hellier, B., Karasev, A. V., Kelly, J. D., McClean, P., Miklas, P., Myers, J. R., Osorno, J. M., Pasche, J. S., Pastor-Corrales, M. A., Porch, T., Steadman, J. R., Urrea, C., Wallace, L., Diepenbrock, C. H., Gepts, P. (2023). Genetic resources and breeding priorities in Phaseolus beans: vulnerability, resilience and future challenges. Plant Breeding Reviews 46, 6, 289-417. https://doi.org/10.1002/9781119874157.ch6.
Parsons, L. R., Howe, T. K. (1984). Effects of water stress on the water relations of Phaseolus vulgaris and the drought resistant Phaseolus acutifolius. Physiol. Plant. 60, 2, 197-202.
Porch, T., Beaver, J., Arias, J., Godoy-Lutz, G. (2021). Response of tepary beans to Bean Golden Yellow Mosaic Virus and powdery mildew. Annu. Rep. Bean Improvem. Coop. (USA) 64, 73-74.
Porch, T., Estévez de Jensen, C. (2024). Response of the tepary diversity panel to combined Asian bean flower thrip and leafhopper pressure. Annu. Rep. Bean Improvem. Coop. (USA) 67, 117-118.
Porch, T. G., Rosas, J. C., Cichy, K., Godoy Lutz, G., Rodríguez, I., Colbert, R. W., Demosthene, G., Hernández, J. C., Winham, D. M., Beaver, J. S. (2024). Release of tepary bean cultivar ‘USDA Fortuna’ with improved disease and insect resistance, seed size, and culinary quality. J. Plant Registrations 18, 1, 42-51. https://doi.org/10.1002/plr2.20322.
Pratt, R. C., Grant, L., Velasco-Cruz, C., Lauriault, L. (2023). Field performance of selected and landrace tepary bean varieties in diverse southwestern USA irrigated production environments. Legume Sci. 5, e157, 1-8. .
Pratt, R. C., Nabhan, G. P. (1988). Evolution and diversity of Phaseolus acutifolius genetic resources. In Genetic resources of Phaseolus beans, ed. P. Gepts (Dordrecht, Holland: Kluwer Academic Publishers), 409-440.
Reichenbacher, F. W., Peachey, W. D. (2025). Cyclic interannual variation in monsoon onset and rainfall in South Central Arizona, USA. Climate 13, 75, 1-21. https://doi.org/10.3390/cli13040075.
Rocha, G., Le Queré, A., Medina, A., Cuéllar, A., Contreras, J-L., Carreño, R., Bustillos, R., Muñoz-Rojas, J., Villegas, M. C., Chaintreuil, C., Dreyfus, B., Munive, J.-A. (2020). Diversity and phenotypic analyses of salt- and heat-tolerant wild bean Phaseolus filiformis native of a sand beach in Baja California and description of Ensifer aridi sp. nov. Archiv. Microbiol. 202, 2, 309-322. https://doi.org/10.1007/s00203-019-01744-7.
Schmutz, J., McClean, P. E., Mamidi, S., Wu, G. A., Cannon, S. B., Grimwood, J., Jenkins, J., Shu, S., Song, Q., Chavarro, C., Torres-Torres, M., Geffroy, V., Moghaddam, S. M., Gao, D., Abernathy, B., Barry, K., Blair, M., Brick, M. A., Chovatia, M., Gepts, P., Goodstein, D. M., Gonzales, M., Hellsten, U., Hyten, D. L., Jia, G., Kelly, J. D., Kudrna, D., Lee, R., Richard, M. M. S., Miklas, P. N., Osorno, J. M., Rodrigues, J., Thareau, V., Urrea, C. A., Wang, M., Yu, Y., Zhang, M., Wing, R. A., Cregan, P. B., Rokhsar, D. S., Jackson, S. A. (2014). A reference genome for common bean and genome-wide analysis of dual domestications. Nature Genetics 46, 7, 707-713. https://doi.org/10.1038/ng.3008.
Shade, R. E., Pratt, R. C., Pomeroy, M. A. (1987). Development and mortality of the bean weevil, Acanthoscelides obtectus (Coleoptera: Bruchidae), on mature seeds of tepary beans, Phaseolus acutifolius, and common beans, Phaseolus vulgaris. Environ. Entomol. 16, 5, 1067-1070. https://doi.org/10.1093/ee/16.5.1067.
Silber-Coats, N., Elias, E., Fernald, K., Gagliardi, M. (2025). Evaluating alternative crops as a solution to water stress in the U. S. Southwest. Agric. Water Manag. 312, 1-15 (109439). https://doi.org/10.1016/j.agwat.2025.109439.
Singh, S., Mishra, V., Riyazzudin, R., Chugh, V., Upadhyay, S. K. (2024). CRISPR/Cas9-mediated genome editing for trait improvement and stress tolerance in Leguminosae (Legume family). Plant Breeding 0, 1-19. https://doi.org/10.1111/pbr.13234.
Souter, J. R., Gurusamy, V., Porch, T. G., Bett, K. E. (2017). Successful introgression of abiotic stress tolerance from wild tepary bean to common bean. Crop Sci. 57, 3, 1160-1171. https://doi.org/10.2135/cropsci2016.10.0851.
Thiers, B. M. (2023). [continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium, New York, USA. http://sweetgum.nybg.org/science/ih/. Accessed on 2 January 2023 and verified on 19 July 2023.
Van Devender, T. R., Avila-Villegas, S., Emerson, M., Turner, D., Flesch, A. D., Deyo, N. S. (2013). Biodiversity in the Madrean archipelago of Sonora, Mexico. In Merging science and management in a rapidly changing world: biodiversity and management of the Madrean Archipelago III, ed. G. J. Gottfried, P. F. Ffolliott, B. S. Gebow, L. G. Eskew and L. C. Collins (Fort Collins, Colorado, USA: USDA Forest Service, Rocky Mountain Research Station Proceedings) 67, 10-16.
Voysest, O., Dessert, M. (1991). Bean cultivars: classes and commercial seed types. In Common beans: research for crop improvement, ed. A. van Schoonhoven and O. Voysest (Wallingford, United Kingdom: Commonwealth Agricultural Bureaux International), 119-162. ISBN: 0-85198-679-X.
Wang, Y-W., Wood, J. C., Hamilton, J. P., Mailloux, K., Vaillancourt, B., Estévez de Jensen, C., Porch, T., Buell, C. R. (2024). Genome-enabled breeding across Phaseolus species. Annu. Rep. Bean Improvem. Coop. (USA) 67, 57-58.
Williams, A. P., E.R. Cook, E. R., Smerdon, J. E., Cook, B. I., Abatzoglou, J. T., Bolles. K., Baek, S. H., Badger, A. M., Livneh, B. (2020). Large contribution from anthropogenic warming to an emerging North American megadrought. Science 368, 314-318. https://doi.org/10.1126/science.aaz9600.
Wooton, E. O., Standley, P. C. (1915). Flora of New Mexico. Contr. US Natl. Herb. 19, 9-794.
Zink, D., Nagl, W. (1998a). A taxon identified by microsatellite-primed PCR and Southern hybridization in the secondary gene pool of the tepary bean. Annu. Rep. Bean Improv. Coop. (USA) 41, 107-108.
Zink, D., Nagl, W. (1998b). Interspecific microsatellite-primed PCR analysis in 20 different Phaseolus species. Annu. Rep. Bean Improv. Coop. (USA) 41, 109-110.
1 ARIZ, University of Arizona, USA; F, Field Museum of Natural History, USA; ISC, Iowa State University, USA; MO, Missouri Botanical Garden, USA; NMC, New Mexico State University, USA; NY, New York Botanical Garden, USA
2 CAS, California Academy of Sciences, USA; K, Royal Botanic Garden, Kew, UK; L, Naturalis Biodiversity Center, The Netherlands; MIN, University of Minnesota, USA