Abstract
Despite the high variability of Argentine maize (Zea mays L.) landraces, they are scarcely used by breeders due to the limited knowledge available about the genetic merit of these materials. In this study, we evaluated agro-morphological and molecular traits of 36 landraces of the ‘Cristalino Colorado’ race from Buenos Aires province, Argentina. Fifteen agro-morphological traits and five polymorphic microsatellite markers located on different chromosomes (48 alleles) were used. A principal component analysis was performed using average values of agro-morphological traits across two environments. Molecular markers were subjected to a principal coordinate analysis. A generalized procrustes analysis was used to evaluate agro-morphological and molecular traits together, showing seven groups. Distance between agro-morphological and molecular data had an average value of 0.24 and the range varied between 0.02 (ARZM01017) and 0.45 (ARZM01082). The results show that Argentine landraces of the ‘Cristalino Colorado’ race are a valuable source of new alleles for crop improvement. Studies of this type facilitate the selection of landraces for introduction in genetic breeding programmes and for the establishment of core collections.
Keywords: Genetic variability, Generalized Procrustes Analysis, SSR markers, agro-morphological traits, landraces
Introduction
Maize (Zea mays L.) landraces originated from long-term cultivation under natural and artificial selection in different environments and under different cultural management schemes (Xiang et al, 2010). These landraces maintain high genetic variation and good adaptation to the natural and anthropological environment where they have evolved (Lucchin et al, 2003; Di Pasquale et al, 2024). The mechanization of agriculture, the increase of urban areas, changes in consumption patterns and production systems has led to the replacement of landraces by improved varieties or hybrids (Pilling et al, 2020). Mechanization of agriculture and new market demands forced breeders to generate more uniform and productive crops with stable yield (Esquinas Alcázar, 2005). This homogeneity resulted in an irreversible loss of genetic variability, known as genetic erosion, with the consequent increase of the vulnerability of agricultural crops to future attack by biotic and abiotic stresses (Salhuana et al, 1998; Troyer et al, 1988; Esquinas Alcázar, 2005).
Genetic resources have long been an important source of new alleles for commercial plant breeding. However, high variability conserved in germplasm banks worldwide is poorly used because breeders prefer crosses among elite inbred lines for their improvement programmes (Vigouroux et al, 2008). Extensive exploitation of landraces is hampered by their high heterogeneity, low performance, seed underproduction and negative genetic load (Gorjanc et al, 2016).
Using conserved germplasm in future plant breeding requires systematic evaluation of desired traits (Xiang et al, 2010; Balconi et al, 2024). For this purpose, there are many descriptors, such as agro-morphological traits and molecular markers, which contribute different and equally important information.
Germplasm characterization and evaluation must be complemented with appropriate statistical analyses to obtain a more complete description of the landraces and establish relations among them. Properly studied and catalogued plant genetic resources can be available for plant breeding programmes (Bramardi, 2023).
In Argentina, as in other countries of the Americas, there is a large diversity of maize types. Argentine maize landraces are classified into 44 races mainly based on specific traits related to ear and grain descriptors, such as shape, colour and texture, and use (Cámara Hernández and Miante Alzogaray, 2003; Solari, 2007). Argentina has a leading role in the production of export maize ‘Plata’, typical of the ‘Cristalino Colorado’ race, being currently the only producer of this type of maize worldwide. The kernel of ‘Cristalino Colorado’ maize is of intense orange colour; the endosperm is mostly hard and glassy in the periphery and floury in the centre, lacking indentation (Secretaría de Agricultura, Ganadería, Pesca y Alimentación, 1997). This kind of grain is widely used in dry milling processes for human consumption and poultry feeding. This grain contains higher carotenoid concentrations than dent corns (Chandler et al, 2013); when included in the diet of chicken, it gives a desirable colour to the skin and egg yolks, without the addition of synthetic pigments. Besides, true metabolizable energy values of ‘Cristalino Colorado’ maize are higher than those of the dent maize due to the higher concentration of oil in the grain. ‘Cristalino Colorado’ maize can supply the calories required by cattle and pigs, with no need for additional oil in their diet. A ‘Cristalino Colorado’ hybrid is available in the Argentine market; however, it is of lower quality than traditional genotypes (Paz, 2009).
Previous studies of maize landraces conserved in the Active Germplasm Bank at ‘Instituto Nacional de Tecnología Agropecuaria’ (INTA) Pergamino (BAP) revealed a high degree of molecular and agro-morphological variability (Salhuana et al, 1998; Defacio et al, 2005; Paz et al, 2005; Defacio, 2009; Paz, 2009; Defacio, 2017; Heck et al, 2020; Rivas et al, 2022), as well as in disease resistance (Presello et al, 1996; Presello et al, 2006; Iglesias, 2008; Defacio et al, 2018) and grain quality traits (López et al, 2005; Heck et al, 2019). The aim of the present study was to characterize the variability of 36 maize landraces of ‘Cristalino Colorado’ race collected from Buenos Aires province, Argentina, based on agro-morphological traits and SSR markers, and the relationship among them.
Materials and methods
Plant material
Thirty-six maize landraces of ‘Cristalino Colorado’ race conserved at BAP were evaluated. These landraces were collected in Buenos Aires province between 1951 and 1963 (Luna and Safont Lis, 1978). Landrace passport descriptors and races are presented in Table 1. Four synthetic open-pollinated (OP) varieties developed by the INTA Pergamino corn breeding programme, Payagua INTA, Candelaria INTA, SP1234 and BS13p, were included as checks. Payagua INTA and Candelaria INTA have semi-dent endosperm and SP1234 belongs to the ‘Cristalino Colorado’ race. BS13p has dent endosperm and was developed through recurrent selection applied to BS13.
Table 1. Landrace passport descriptors and races.
|
Identifier |
Location |
Department |
Altitude (masl) |
Latitude |
Longitude |
Race |
|
ARZM01001 |
Acevedo |
Pergamino |
70 |
33°46’ S |
60°27’ W |
Cristalino Colorado |
|
ARZM01002 |
Rancagua |
Pergamino |
69 |
34°02’ S |
60°30’ W |
C. Colorado – C. Amarillo |
|
ARZM01003 |
Rancagua |
Pergamino |
69 |
34°02’ S |
60°30’ W |
Cristalino Colorado |
|
ARZM01005 |
Arroyo Dulce |
Salto |
75 |
34°06’ S |
60°24’ W |
Cristalino Colorado |
|
ARZM01006 |
Tacuarí |
Salto |
69 |
34°13’ S |
60°19’ W |
C. Colorado – C. Amarillo |
|
ARZM01007 |
Salto |
Salto |
51 |
34°18’ S |
60°15’ W |
Cristalino Colorado |
|
ARZM01008 |
Salto |
Salto |
51 |
34°18’ S |
60°15’ W |
Cristalino Colorado |
|
ARZM01012 |
Arenales |
General Arenales |
84 |
34°19’ S |
61°18’ W |
C. Colorado – C. Amarillo |
|
ARZM01013 |
Rojas |
Rojas |
69 |
34°12’ S |
60°44’ W |
C. Colorado – C. Amarillo |
|
ARZM01014 |
Chacabuco |
Chacabuco |
69 |
34°38’ S |
60°29’ W |
Cristalino Colorado |
|
ARZM01015 |
Salto |
Salto |
51 |
34°18’ S |
60°15’ W |
Cristalino Colorado |
|
ARZM01016 |
Arroyo Burgos |
Bartolomé Mitre |
|
34°04’ S |
60°07’ W |
Cristalino Colorado |
|
ARZM01017 |
San Pedro |
San Pedro |
27 |
33°42’ S |
59°41’ W |
C. Colorado – C. Amarillo |
|
ARZM01022 |
Ortiz Basualdo |
Pergamino |
64 |
34°03’ S |
60°39’ W |
C. Colorado |
|
ARZM01025 |
Hunter |
Rojas |
50 |
34°15’ S |
60°32’ W |
C. Colorado – C. Amarillo |
|
ARZM01026 |
Ferré |
General Arenales |
88 |
34°08’ S |
61°08’ W |
Cristalino Colorado |
|
ARZM01027 |
Carabelas |
Rojas |
83 |
34°03’ S |
60°52’ W |
Cristalino Colorado |
|
ARZM01028 |
Colón |
Colón |
90 |
33°59’ S |
61°06’ W |
Cristalino Colorado |
|
ARZM01030 |
Conesa |
San Nicolás |
58 |
33°36’ S |
60°22’ W |
Cristalino Colorado |
|
ARZM01033 |
El Paraíso |
Ramallo |
33 |
33°34’ S |
59°59’ W |
C. Colorado – C. Amarillo |
|
ARZM01036 |
Conesa |
San Nicolás |
58 |
33°36’ S |
60°22’ W |
Cristalino Colorado |
|
ARZM01039 |
Rancagua |
Pergamino |
69 |
34°02’ S |
60°30’ W |
Cristalino Colorado |
|
ARZM01044 |
Chivilcoy |
Chivilcoy |
55 |
34°54’ S |
60°01’ W |
Cristalino Colorado |
|
ARZM01048 |
La Violeta |
Pergamino |
55 |
33°44’ S |
60°11’ W |
Cristalino Colorado |
|
ARZM01058 |
Chivilcoy |
Chivilcoy |
55 |
34°54’ S |
60°01’ W |
C. Colorado – C. Amarillo |
|
ARZM01062 |
Chacabuco |
Chacabuco |
69 |
34°38’ S |
60°29’ W |
C. Colorado – Amarillo Ocho Hileras |
|
ARZM01066 |
Rojas |
Rojas |
69 |
34°12’ S |
60°44’ W |
Cristalino Colorado |
|
ARZM01082 |
Nueva Roma |
Torquinst |
285 |
38°06’ S |
62°14’ W |
Cristalino Colorado |
|
ARZM01086 |
Nueva Roma |
Torquinst |
285 |
38°06’ S |
62°14’ W |
Cristalino Colorado |
|
ARZM01087 |
Nueva Roma |
Torquinst |
285 |
38°06’ S |
62°14’ W |
Cristalino Colorado |
|
ARZM01092 |
Pigüé |
Saavedra |
298 |
37°41’ S |
62°24’ W |
Cristalino Colorado |
|
ARZM01096 |
Coronel Suárez |
Coronel Suárez |
234 |
37°28’ S |
61°56’ W |
Cristalino Colorado |
|
ARZM01102 |
Carhue |
Adolfo Alsina |
112 |
37°11’ S |
62°45’ W |
Cristalino Colorado |
|
ARZM01124 |
Trenque Lauquen |
Trenque Lauquen |
96 |
35°58’ S |
62°44’ W |
Cristalino Colorado |
|
ARZM01151 |
Mones Cazón |
Pehuajó |
88 |
35°48’ S |
61°53’ W |
Cristalino Colorado |
|
ARZM01152 |
Carlos Tejedor |
Carlos Tejedor |
96 |
35°23’ S |
62°25’ W |
Cristalino Colorado |
Agro-morphological characterization
Evaluations were performed in two environments, Pergamino (33°53′01″ S, 60°34′01″ W) and Ferré (34°07’30” S, 61°08’27” W), Buenos Aires province, Argentina, during the 2004/2005 growing season. Both are characterized by typical Argiudol soil, (INTA, 1972). The climate is classified as humid temperate, characterized by annual average rainfall of 1,000mm and average temperature of 16–18 ºC. Figure 1 presents meteorological data recorded as long-term averages (1982–2005) and during the 2004/2005 growing season for the two environments. Minimum and maximum temperatures and long-term precipitation data were obtained from the NASA POWER package in R (Sparks, 2018), while precipitation during the 2004/2005 growing season was manually recorded in the field.
Both trials were conducted using a randomized complete block design with two replications. Each plot was planted in two 5m rows with a spacing of 70cm and 30 plant hills. Standard agronomic practices were followed for successful crop growth.
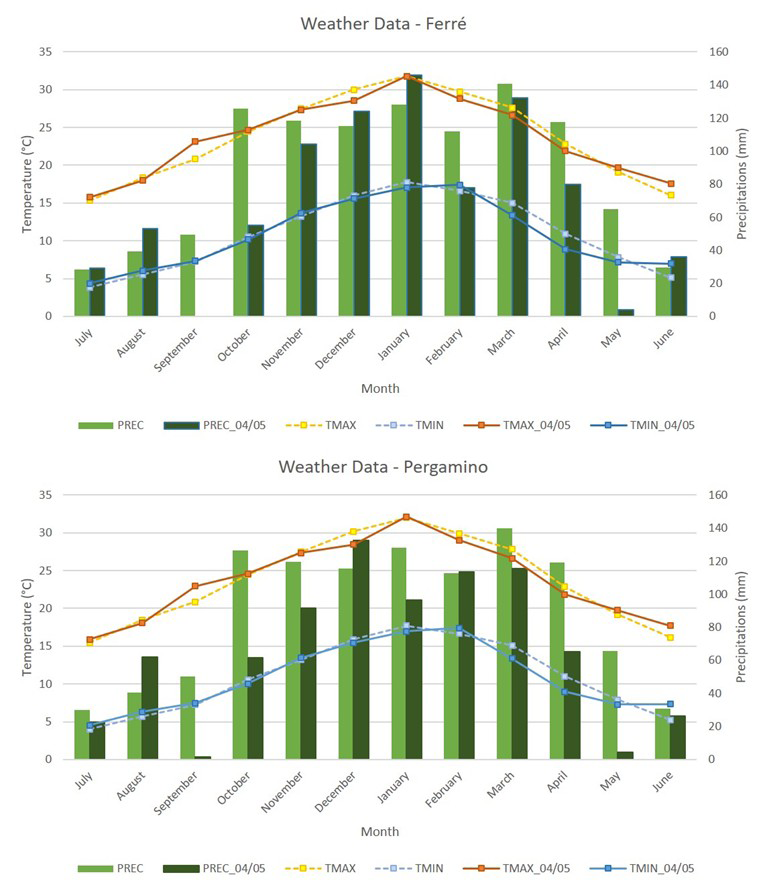
Figure 1. Meteorological data recorded as long-term averages and during the 2004/2005 growing season in Ferré (a) and Pergamino (b), Buenos Aires province, Argentina. PREC, long-term precipitations; PREC_04/05, precipitations in 2004/2005 growing season; TMAX, long-term maximum temperatures; TMAX_04/05, maximum temperatures in 2004/2005 growing season; TMIN, long-term minimum temperatures; TMIN_04/05, minimum temperatures in 2004/2005 growing season.
Fifteen quantitative traits, based on maize descriptor (CIMMYT/IBPGR, 1991) were evaluated: days to anthesis (GDU, growing degree units), days to silk (GDU), anthesis-silking interval (GDU), ear length (cm), ear diameter (mm), number of kernel rows (number), kernels per row (number), kernel width (mm), kernel length (mm), plant height (cm), ear height (cm), plant height/ear height ratio (index), 1,000-kernel weight (g), yield (kg/ha), and prolificacy (index). Phenological traits, 1000-kernel weight, yield and prolificacy were measured on the complete plot. Morphological traits were collected on ten plants per plot, randomly selected in each plot, using the average of the 10 units for performing the analyses. A principal component analysis (PCA) was performed using the standardized data matrix obtained from the arithmetic means of the agro-morphological quantitative variables corresponding to both environments and replications, in order to obtain an average characterization throughout the environments (Zuliani et al, 2018). Pearson correlation was computed to assess the relationship among traits prior to PCA.
Molecular characterization
Landraces were evaluated using a set of five public SSR markers (phi080, phi072, phi034, bnlg439 and phi96100) located on different chromosomes, with a high degree of polymorphism. Oligonucleotide sequences are publicly available at the Maize Data Bank (www.agron.missouri.edu/Coop/SSR-Probes/SSR1.html). DNA was extracted from young leaves of 25 plants per landrace, according to Kleinhofs et al (1993). PCR reactions were carried out in a MJ Research PTC-100 thermocycler (USA). The amplification products were visualized in 6% polyacrylamide gels and were detected by silver nitrate staining (Benbouza et al, 2006). The relative allelic frequencies were calculated using the direct counting method from the individual genotypes found in each landrace.
The Prevosti distance (Prevosti, 1974) was calculated from the relative allelic frequencies of the molecular markers for each landrace to infer the relationship between landraces using a principal coordinate analysis (PCoA). The allele number was determined for each group.
Subsequently, expected heterozygosity (He) was calculated for each group identified in the PCoA, which was calculated using the following equation:
k
He = 1 - ∑ p i 2
i=1
where, pi is the frequency of the i-th allele, and k is the number of alleles.
Joint analysis
For joint analysis, generalized procrustes analysis (GPA) (Gower, 1975) was performed. This method gets a better adjustment for the information provided by both PCA and PCoA. A consensus configuration was performed and represented in a two-dimensional space. A minimum spanning tree (MST) from the Euclidean matrix obtained from the first two GPA coordinates was added in the principal plane.
A Mantel test was performed to quantify the relationship between the molecular, agro-morphological and consensus distance matrix.
To determine concordance of molecular and agro-morphological characterizations at the landrace level, we calculated the Euclidean distance between two analogous points, i.e. points corresponding to a single landrace in the agro-morphological and molecular configurations.
All statistical analyses were carried out using the NTSYS programme (Numerical Taxonomic System ver. 2.11) (Rohlf, 2002).
Results and discussion
Agro-morphological characterization
Table 2 presents the mean values, standard errors, and ranges for each trait, calculated across landraces in each environment under evaluation.
Table 2. Mean values, standard errors (S.E.), and ranges for each trait evaluated in two environments.
|
Environment |
Pergamino 2004/2005 |
Ferré 2004/2005 |
||||
|
Trait |
Mean |
S.E. |
Range |
Mean |
S.E. |
Range |
|
Ear length (cm) |
15.99 |
0.14 |
13.10–19.50 |
16.56 |
0.11 |
14.00–18.70 |
|
Ear diameter (mm) |
41.46 |
0.31 |
33.40–48.70 |
42.04 |
0.30 |
34.50–48.10 |
|
Number of kernel rows |
13.16 |
0.17 |
10 .00–16.60 |
13.09 |
0.15 |
9.80–16.40 |
|
Kernel width (mm) |
8.48 |
0.09 |
6.00–9.80 |
8.77 |
0.07 |
7.20–10.20 |
|
Kernel length (mm) |
8.19 |
0.12 |
5.40–12.00 |
10.11 |
0.10 |
8.00–12.00 |
|
Kernels per row |
31.25 |
0.40 |
21.60–38.40 |
34.90 |
0.28 |
29.90–40.00 |
|
Prolificacy (index) |
0.94 |
0.02 |
0–1.36 |
1.01 |
0.01 |
0.66–1.33 |
|
1,000-kernel weight (g) |
281.30 |
3.78 |
174.00–355.00 |
285.67 |
3.31 |
201.00–346.00 |
|
Yield (Kg/ ha) |
5576.90 |
176.80 |
1341.10–9440.80 |
6838.30 |
172.90 |
2623.80–11561.30 |
|
Plant height (cm) |
163.98 |
1.56 |
122.50–193.00 |
143.42 |
1.18 |
118.50–165.50 |
|
Ear height (cm) |
96.88 |
1.22 |
68.00–117.50 |
84.20 |
1.10 |
65.00–106.50 |
|
Plant height/ear height ratio (index) |
1.70 |
0.01 |
1.52–2.03 |
1.71 |
0.01 |
1.43–2.09 |
|
Days to anthesis (GDU) |
1032.30 |
3.86 |
944.70–1100.15 |
903.23 |
3.49 |
837.60–1017.55 |
|
Days to silking (GDU) |
1069.75 |
3.09 |
991.10–1143.75 |
974.92 |
5.10 |
895.90–1089.80 |
|
Anthesis-silking interval (GDU) |
37.45 |
1.79 |
12.80–108.12 |
71.69 |
2.91 |
15.00–155.30 |
In the Ferré 2004/2005 growing season, plants exhibited shorter height, a shorter anthesis-silking interval, and higher grain yields. These differences may be attributed to the higher precipitation levels recorded in Ferré during January, which match with the critical period for determining grain yield (30 days centred around flowering (Fischer and Palmer, 1984)). Maize is particularly sensitive during this stage, and any stress can increase the anthesis-silking interval, potentially leading to pollination failure and grain yield loss (Tao et al, 2023). The other traits did not show differences between the two environments.
The correlation matrix (Table 3) revealed strong and significant (p < 0.01) correlations between several traits: days to silking and days to anthesis (r = 0.88), plant height and ear height (r = 0.87), yield and ear diameter (r = 0.79), ear height and plant height/ear height (r = -0.78), yield and ear length (r = 0.74), and kernel length and ear diameter (r = 0.73). Similar correlations among these traits have been reported by other authors during the evaluation of maize landraces (Defacio, 2009; Javed et al, 2021; de Faria et al, 2022).
Table 3. Correlation matrix between evaluated traits. EL, ear length; ED, ear diameter; NKR, number of kernel rows; KW, kernel width; KL, kernel length; KR, kernels per row; HKW, 1,000-kernel weight; PH, plant height; EH, ear height; PH/EH, plant height/ear height ratio; PROL, prolificacy; DA, days to anthesis; DS, days to silking; ASI, anthesis–silking interval; NS, non-significant (p > 0.05); *, significant at p < 0.05; **, significant at p < 0.01.
|
|
EL |
ED |
NKR |
KW |
KL |
KR |
HKW |
Yield |
PH |
EH |
PH/ EH |
PROL |
DA |
DS |
ASI |
|
EL |
1 |
** |
NS |
NS |
** |
** |
** |
** |
** |
NS |
* |
NS |
NS |
NS |
NS |
|
ED |
0.56 |
1 |
** |
NS |
** |
NS |
** |
** |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
NKR |
0.12 |
0.49 |
1 |
** |
* |
NS |
NS |
* |
* |
NS |
NS |
NS |
NS |
NS |
NS |
|
KW |
0.16 |
-0.08 |
-0.46 |
1 |
NS |
NS |
* |
NS |
** |
* |
NS |
* |
NS |
NS |
NS |
|
KL |
0.51 |
0.73 |
0.36 |
0.10 |
1 |
NS |
** |
** |
NS |
NS |
NS |
NS |
NS |
NS |
NS |
|
KR |
0.61 |
0.10 |
-0.17 |
0.18 |
0.26 |
1 |
NS |
NS |
* |
NS |
NS |
NS |
NS |
NS |
NS |
|
HKW |
0.62 |
0.64 |
0.09 |
0.38 |
0.66 |
0.11 |
1 |
** |
* |
NS |
NS |
* |
NS |
NS |
NS |
|
Yield |
0.74 |
0.79 |
0.34 |
0.05 |
0.70 |
0.27 |
0.75 |
1 |
NS |
NS |
NS |
** |
NS |
NS |
NS |
|
PH |
0.46 |
0.15 |
-0.32 |
0.50 |
0.20 |
0.40 |
0.43 |
0.28 |
1 |
** |
* |
NS |
NS |
NS |
NS |
|
EH |
0.23 |
0.02 |
-0.28 |
0.41 |
0.11 |
0.27 |
0.26 |
0.11 |
0.87 |
1 |
** |
NS |
* |
* |
NS |
|
PH/EH |
0.14 |
0.11 |
0.10 |
-0.13 |
0.03 |
-0.02 |
0.04 |
0.11 |
-0.37 |
-0.78 |
1 |
NS |
** |
** |
NS |
|
PROL |
0.27 |
0.24 |
-0.16 |
0.33 |
0.27 |
0.10 |
0.35 |
0.44 |
0.22 |
0.07 |
0.14 |
1 |
NS |
NS |
NS |
|
DA |
-0.04 |
-0.03 |
-0.07 |
0.06 |
0.18 |
0.09 |
-0.11 |
-0.11 |
0.25 |
0.43 |
-0.50 |
-0.01 |
1 |
** |
NS |
|
DS |
-0.02 |
0.04 |
-0.09 |
0.11 |
0.10 |
-0.02 |
-0.04 |
-0.09 |
0.29 |
0.43 |
-0.45 |
0.10 |
0.88 |
1 |
** |
|
ASI |
0.04 |
0.15 |
-0.06 |
0.12 |
-0.13 |
-0.21 |
0.13 |
0.03 |
0.14 |
0.10 |
-0.01 |
0.22 |
-0.03 |
0.45 |
1 |
Results from the PCA (Figure 2) show that the first and second principal components (PC1 and PC2, respectively) accounted for 27.42% and 20.86% of the total variation, respectively. The axis loadings corresponding to PC1 and PC2 are shown in Table 4. PC1 was positively and strongly associated with yield, 1000-kernel weight, kernel length, ear diameter and ear length. PC2 was negatively and moderately associated with plant architecture traits (plant height and ear height), days to anthesis, and days to silking.
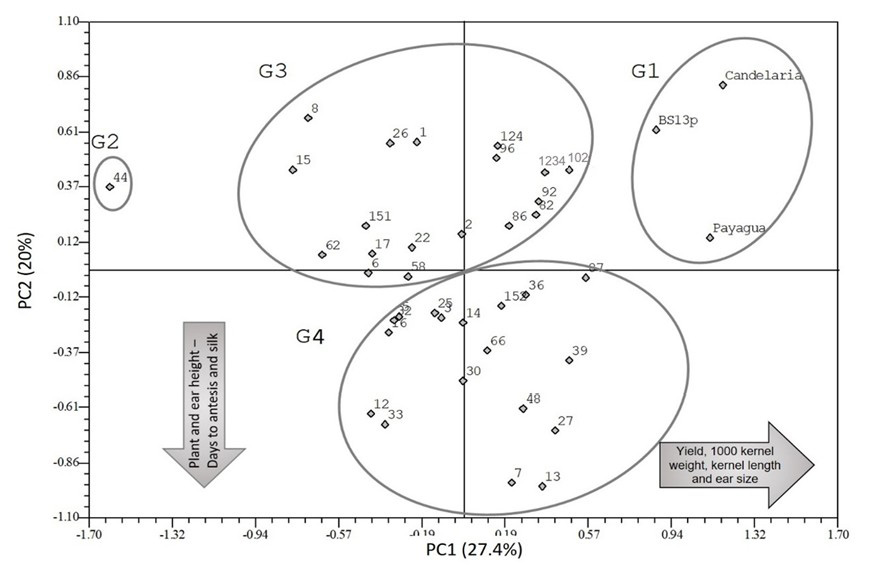
Figure 2. Principal Component Analysis of agro-morphological traits. Landraces are identified with the last numbers in their identifier (e.g. 13 corresponds to ARZM1013).
Table 4. Axis loadings corresponding to PC1 and PC2
|
Trait |
PC1 |
PC2 |
|
Ear lenght |
0.8324 |
0.0588 |
|
Ear diameter |
0.7851 |
0.3346 |
|
Number of kernel rows |
0.2442 |
0.6234 |
|
Kernel width |
0.2345 |
-0.5806 |
|
Kernel lenght |
0.7884 |
0.1752 |
|
Kernels per row |
0.4068 |
-0.2193 |
|
Prolificacy |
0.3596 |
-0.1912 |
|
1,000-kernel weight |
0.8402 |
0.0398 |
|
Yield |
0.8861 |
0.2641 |
|
Plant height |
0.5499 |
-0.6681 |
|
Ear height (cm) |
0.3948 |
-0.7835 |
|
Plant height/ear height ratio |
-0.0741 |
0.6170 |
|
Days to antesis |
0.1024 |
-0.5616 |
|
Days to silking |
0.1209 |
-0.6186 |
|
Anthesis-silking interval |
0.0623 |
-0.2467 |
Landraces were classified in four groups by PCA, based on the distances observed between individuals in the direction of both established gradients.
G1. This group included BS13p, Candelaria INTA and Payagua INTA. These OP varieties were associated with the highest values of yield and its components, as well as shorter plant and ear height and fewer days to anthesis and silking than the rest of the evaluated landraces. This result agrees with the fact that these genotypes were selected for yield purposes.
G2. Represented by only one accession (ARZM01044) that showed the lowest yield and the smallest ear, grain size and 1,000-kernel weight of all landraces. This accession presented medium to low plant height and intermediate to fewer days to anthesis and silking.
G3. This group included landraces with average yield, 1,000-kernel weight, kernel length, ear diameter, and ear length, displaying shorter plants, lower ear height and fewer days to anthesis and silking than the G4 cluster.
G4. Represented by landraces with average yield, 1,000-kernel weight, kernel length, ear diameter and ear length, and highest values for days to anthesis and silking, high plant and ear height.
Molecular characterization
In this study, a set of five SSR markers was employed for the preliminary molecular characterization of maize landraces. Other authors (Di Pasquale et al, 2024; Joshi et al, 2020; Ignjatović Micić et al. 2013) have also used a low number of SSRs, ranging from 5 to 10, to evaluate maize landraces.
A total of 48 alleles were detected. The overall number of alleles per locus varied from 6 (phi034 and phi072) to 21 (bnlg439), with an average of 9.6 (Table 5). Six alleles were unique to landraces (unique or private alleles) while five other different alleles were present in two landraces (rare alleles).
Table 5. Numbers of alleles per locus across landraces
|
SSR markers |
No. of alleles |
|
phi080 |
8 |
|
phi072 |
6 |
|
phi034 |
6 |
|
phi96100 |
7 |
|
bnlg439 |
21 |
|
Average |
9.6 |
The average number of alleles per locus obtained from landraces (9.6) was higher than the values reported by Reif et al (2003) (5.9), Warburton et al (2002) (6.3), Labate et al (2003) (6.5) and Di Pasquale et al (2024) (7.4), but lower than those reported by Barcaccia et al (2003) (20.75), Rivas et al (2022) (19.05) and Torres-Morales et al (2023) (25.39).
In the OP varieties, the assayed loci scored a mean number of alleles equal to 7.6, lower than landraces (9.6). This result is consistent with that obtained by Barcaccia et al (2003) of 10.25 vs. 19.75, showing that even though OP varieties have genetic variability, they originated from a narrow genetic base.
First and second principal axes of PCoA (Figure 3) accounted for 10.79 and 8.96% of the total variation, respectively. Landraces were distributed in four groups based on their relative distances on the principal plane, which differed from those obtained using PCA.
G1. Represented by ARZM01082 and ARZM01086 landraces.
G2. This group included ARZM01124, ARZM01102, ARZM01152, BS13p and Candelaria INTA.
G3 and G4. These groups included most of the evaluated landraces and were separated by the dispersion of the second principal coordinate (PCO2).
Figure 3. Principal coordinate analysis of molecular traits. Landraces are identified with the last numbers in their identifier (e.g. 13 corresponds to ARZM1013).
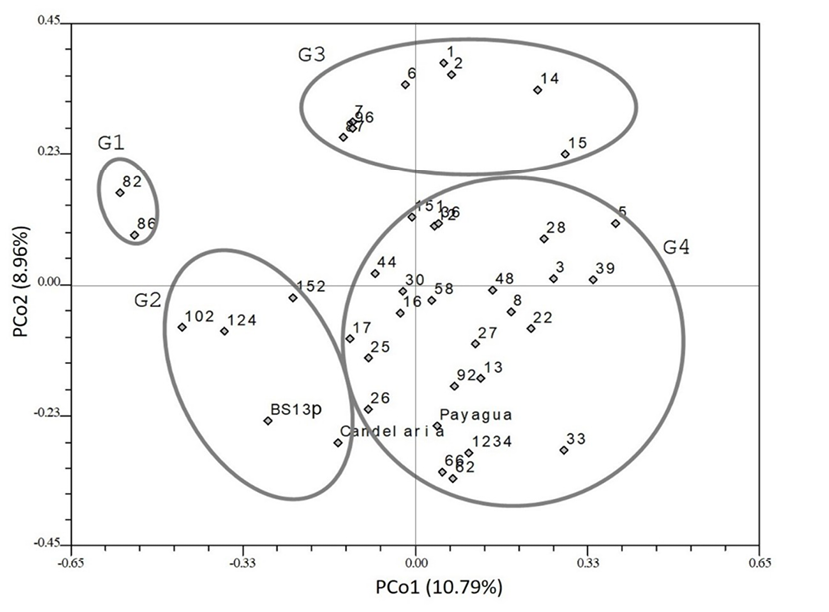
This clustering was not associated with the presence of rare or private alleles. Some landraces exhibited rare or private alleles (ARZM01003, ARZM01049, ARZM01102 and SP1234) but were clustered with other landraces.
Allele numbers for five SSR markers and expected heterozygosity were calculated for each group identified by the PCoA (Figure 4).
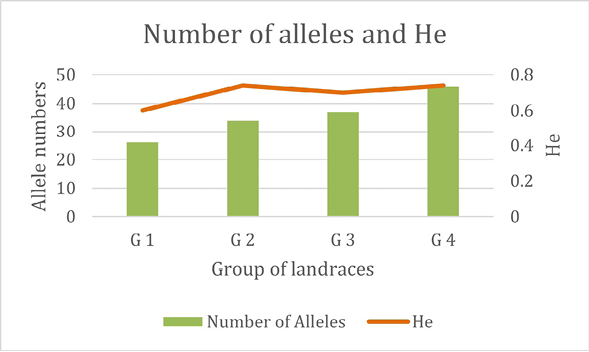
Figure 4. Allele numbers for five SSR markers and expected heterozygosity (He) for each group identified by the PCoA
The number of alleles observed varied among groups, increasing from G1 to G4 in parallel with the number of landraces included in each group. This pattern suggests greater genetic variability in G4, in concordance with the broader dispersion of landraces observed for this group in the PCoA (Figure 3). The expected heterozygosity (He) also varied among groups, but no clear relationship was observed with the total number of alleles.
Joint analysis
The correlation between agro-morphological and molecular data matrices was very low and not significant (r = 0.07, p-value = 0.77). The different configurations obtained with both types of variables indicate that individual characterization offers additional information that can be used complementarily to know the genetic variability among landraces. Low correlation values between agronomic and molecular traits were found in 41 varieties of cucumber (Cucumis sativus L.) (Bramardi et al, 2005), in 37 Patagonian isolates of yeast (Saccharomyces cerevisiae) (Lopes et al, 2006), in 57 red clover landraces (Trifolium pratense L) (Dias et al, 2008), and in a set of banana (Musa sp.) clones (Ermini et al, 2016). For this reason, it is necessary to use a technique that gathers molecular and agro-morphological information.
According to the GPA results (Figure 5), seven groups of landraces were identified.
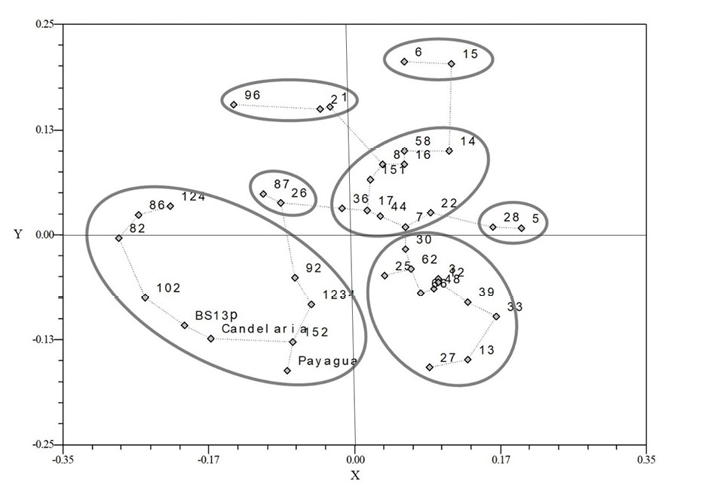
Figure 5. Generalized procrustes analysis of agro-morphological and molecular traits in the first two axis with Minimum Spanning Trees. Landraces are identified with the last numbers in their identifier (e.g. 13 corresponds to ARZM1013).
Some landraces denoted a high correspondence between molecular and agro-morphological characterizations. However, most landraces denoted a great discordance between agro-morphological and molecular data. Distance between both types of data presents a range between 0.02 (ARZM01017) and 0.45 (ARZM01082), with an average of 0.24 (Table 6). The discrepancies observed between agro-morphological and molecular markers may be explained by the fact that the SSR used in this work are neutral and not linked to the agro-morphological traits under evaluation. Expression of these phenotypic traits is strongly influenced by environmental conditions and the selection performed by farmers according to local preferences. This selection contributed to phenotypic differentiation, which may not be reflected in neutral genomic regions, such as those assessed by SSR markers (Javed et al, 2021).
Table 6. Distance between two analogous points, i.e. points corresponding to a single landrace in the agro-morphological and molecular configurations
|
Identifier |
Distance between molecular and agro-morphological traits |
|
ARZM01001 |
0.26 |
|
ARZM01002 |
0.27 |
|
ARZM01003 |
0.21 |
|
ARZM01005 |
0.27 |
|
ARZM01006 |
0.23 |
|
ARZM01007 |
0.37 |
|
ARZM01008 |
0.24 |
|
ARZM01012 |
0.32 |
|
ARZM01013 |
0.42 |
|
ARZM01014 |
0.10 |
|
ARZM01015 |
0.19 |
|
ARZM01016 |
0.06 |
|
ARZM01017 |
0.02 |
|
ARZM01022 |
0.17 |
|
ARZM01025 |
0.14 |
|
ARZM01026 |
0.30 |
|
ARZM01027 |
0.37 |
|
ARZM01028 |
0.23 |
|
ARZM01030 |
0.15 |
|
ARZM01033 |
0.40 |
|
ARZM01036 |
0.06 |
|
ARZM01039 |
0.31 |
|
ARZM01044 |
0.08 |
|
ARZM01048 |
0.24 |
|
ARZM01058 |
0.11 |
|
ARZM01062 |
0.24 |
|
ARZM01066 |
0.24 |
|
ARZM01082 |
0.45 |
|
ARZM01086 |
0.42 |
|
ARZM01087 |
0.25 |
|
ARZM01092 |
0.21 |
|
ARZM01096 |
0.43 |
|
ARZM01102 |
0.26 |
|
ARZM01124 |
0.37 |
|
ARZM01151 |
0.08 |
|
ARZM01152 |
0.23 |
|
BS13P |
0.16 |
|
Candelaria INTA |
0.16 |
|
Payagua INTA |
0.17 |
|
SP 1234 |
0.26 |
Consensus configuration grouped the four OP varieties and the six landraces. Interestingly, ARZM01044 formed a group by itself when evaluated by agro-morphological traits and was included in a group with a larger number of landraces when evaluated by molecular markers and in consensus analysis. ARZM01082 and ARZM01086 landraces were assigned to a separate group, according to molecular markers. However, according to agro-morphological traits, these landraces were grouped with other landraces. In GPA, these landraces were grouped with OP varieties. A similar situation was observed with checks Candelaria INTA, Payagua INTA and BS13p, which formed a distinct group according to agro-morphological analysis but grouped together with other landraces according to the molecular markers and GPA. The correlations among the three distance matrices (molecular, agro-morphological and consensus) were estimated using a Mantel test. A greater correlation was found between the consensus and the molecular and agro-morphological characterization (0.20 and 0.47, respectively) than between molecular and agro-morphological characterization (0.07). This result indicates that GPA allows the simultaneous characterization of a set of accessions with agro-morphological traits and SSR markers. There is no unique pattern of association among landraces, which emphasizes the importance of studying the different descriptors jointly to obtain the best description and interpretation of genetic diversity.
In conclusion, both agro-morphological and molecular variation were detected among the studied landraces, highlighting the importance of integrating both types of characterization to evaluate genetic diversity. GPA is a powerful statistical technique to align genetic and agro-morphological descriptors. Increasing the knowledge of the available genetic diversity in maize germplasm will facilitate the establishment of core collections. Furthermore, integrating agronomic performance with genetic data is critical to developing and optimizing future breeding strategies. Currently, maize breeding relies on a narrow genetic base. Incorporating landraces into crosses with elite varieties offers a promising approach to introduce novel alleles and broaden the genetic base of maize breeding. Moreover, the local adaptation exhibited by landraces represents a valuable source of germplasm for future needs in sustainable agriculture, particularly in the context of climate change.
To enhance the understanding of the genetic diversity of the conserved landraces, it is recommended to incorporate more molecular markers as well as landraces from other races and origins.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Raquel Defacio, Natalia Paz and Sergio Bramardi. The first draft of the manuscript was written by Raquel Defacio and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We are grateful to the staff of Germplasm Bank and Molecular Markers Laboratory at INTA Pergamino for their assistance in field trials and laboratory experiments, respectively. We also thank Dr. Juliana Iglesias for her precious collaboration and valuable suggestions.
This work was financed by the Instituto Nacional de Tecnologia Agropecuaria (INTA) and the Agencia PICTR2002-00109 ‘Conservación, valoración y desarrollo de recursos genéticos vegetales mediante el uso de nuevas tecnologías.’
Data availability statement
Accession-level data are available from the corresponding author upon reasonable request.
Conflict of interest statement
The authors have no competing interests to declare that are relevant to the content of this article.
References
Balconi, C., Galaretto, A., Malvar, R. A., Nicolas, S. D., Redaelli, R., Andjelkovic, V., Revilla, P., Bauland, C., Gouesnard, B., Butron, A., et al (2024) Genetic and Phenotypic Evaluation of European Maize Landraces as a Tool for Conservation and Valorization of Agrobiodiversity. Biology 13, 454. https://doi.org/ 10.3390/biology13060454
Barcaccia, G., Lucchin, M., Parrini, P. (2003) Characterization of a flint maize (Zea mays var. indurata) Italian landraces, II. Genetic diversity and relatedness assessed by SSR and Inter-SSR molecular markers. Genetic Resources and Crop Evolution 50:253-271. https://doi.org/10.1023/A:1023539901316
Benbouza, H., Jacquemin, J. M., Baudoin, J. P., Mergeai, G. (2006) Optimization of a reliable, fast, cheap and sensitive silver staining method to detect SSR markers in polyacrylamide gels. Biotechnologie, Agronomie, Société et Environnement, 10 (2), 77-81.: https://www.researchgate.net/publication/26433489
Bramardi, S. J. (2023) Three-Way Multivariate Analysis for the Characterization of Plant Genetic Resources. Modern Concepts & Developments in Agronomy (MCDA) 12(5): 1223-1227. https://doi.org/10.31031/MCDA.2023.12.000797
Bramardi, S.J., Bernet, G. P., Asíns, M. J., Carbonell, E. A. (2005) Simultaneous Agronomic and Molecular Characterization of Genotypes via the Generalised Procrustes Analysis: An Application to Cucumber. Crop Sci. 45:1603-1609. https://doi.org/10.2135/cropsci2004.0633
Cámara Hernández, J., Miante Alzogaray, A. M. (2003) Caracterización y clasificación, en razas, de maíces nativos de la Provincia de Misiones, Argentina. – IV Simposio de Recursos Genéticos para América Latina y el Caribe. Mar del Plata. Argentina.
Chandler, K., Lipka, A. E., Owens, B. F., Li, H., Buckler, E. S., Rocheford, T., Gore, M. A. (2013) Genetic Analysis of Visually Scored Orange Kernel Color in Maize. Crop Science 53(1):189-200. https://doi.org/10.2135/cropsci2012.02.0129
CIMMYT/IBPGR. 1991. Descriptores de maíz. México-Roma. 88pp.
de Faria, S. V., Zuffo, L. T., Rezende, W. M., Caixeta D. G., Pereira, H. D., Azevedo, C. F., DeLima R. O. (2022) Phenotypic and molecular characterization of a set of tropical maize inbred lines from a public breeding program in Brazil. BMC Genomics 23, 54. https://doi.org/10.1186/s12864-021-08127-7
Defacio, R. A., Hourquescos, M. J., Bramardi, S. J., Ferrer, M. E. (2005) Estudio de variabilidad en poblaciones nativas de maíz. Actas VIII Congreso Nacional de Maíz. Rosario Pp: 383-386.
Defacio, R. A. (2009) Caracterización y evaluación de la variabilidad genética en poblaciones nativas de maíz [Zea mays L.] de la Provincia de Buenos Aires en base a descriptores morfológicos y agronómicos. Tesis Maestría en Genética Vegetal; Área Mejoramiento Genético. UNR/INTA. Magister Scientiae. Marzo, 2009. 93p. https://rephip.unr.edu.ar/handle/2133/13927
Defacio, R. (2017) Evaluación comparativa de distintas estrategias de análisis de datos para la caracterización y ordenamiento de la variabilidad genética de poblaciones locales de maíz (Zea mays L.). Tesis de Doctorado en Ciencias Agrarias. UNR. Agosto 2017. 122p. https://rephip.unr.edu.ar/handle/2133/13925
Defacio, R. A., Iglesias, J., Kistner, M. B, Canteros, F. H., Parrado, J., Ferrer, M. E. (2018) Las poblaciones locales de maíz como fuente para la resistencia a enfermedades. Revista de Tecnología Agropecuaria: 10, 38: 18-21. http://hdl.handle.net/20.500.12123/4381
Di Pasquale, G. M., Stagnati, L., Lezzi, A., Lanubile, A., Marocco, A., Rossi, G., Busconi, M. (2024) Morphological and Genetic Characterization of Maize Landraces Adapted to Marginal Hills in North-West Italy. Plants 13, 1030. https://doi.org/10.3390/plants13071030
Esquinas Alcázar, J. (2005) Protecting crop genetic diversity for food security: political, ethical and technical challenges. Nat Rev Genet 6: 946-953. https://doi.org/10.1038/nrg1729
Ermini, J. L., Tenaglia, G., Pratta, G. R. (2016) Genetic diversity, ancestry relationships and consensus among phenotype and genotype in banana (Musa acuminata) clones from Formosa (Argentina) farmers. Plant Cell Biotechnology and Molecular Biology 17: 267-278. https://www.ikprress.org/index.php/PCBMB/article/view/1486.
Fischer, K. S., Palmer, F. E. (1984) Tropical Maize, In: P. R. Goldsworthy, P. R., Fischer,N. M. Eds., The Physiology of Tropical Field Crops, Wiley, Chichestor, pp. 213- 248.
Gorjanc, G., Jenko, J., Hearne, S. J., Hickey, J. M. (2016) Initiating maize pre-breeding programs using genomic selection to harness polygenic variation from landrace populations. BMC Genomics. 17-30. doi: https://doi.org/10.1186/s12864-015-2345-z.
Gower, J. C. (1975) Generalizad Procrustes Analisys. Physchometrika. 40: 33-51. https://doi.org/10.1007/BF02291478
Heck, M., Defacio, R., Ferrer, M., Cirilo, A., Fariza, S., De Lucia, A., Blaszchik, J. (2019) Evaluación de la calidad nutricional de variedades nativas de maíz de Misiones, Argentina”. Revista de Investigaciones de la facultad de Ciencias Agrarias - UNR, 34. https://doi.org/10.35305/agro34.266
Heck, M., Defacio, R., Ferrer, M., Cirilo, A., Fariza, S., De Lucia, A.., Blaszchik, J. (2020). Evaluación de la variabilidad agromorfológica de poblaciones nativas de maíz de Misiones, Argentina. REevista de ciencia y tecnología 33: 6 - 12. https://www.fceqyn.unam.edu.ar/recyt/index.php/recyt/article/view/264
Iglesias, J. (2008) Potencial de germoplasma nativo de maíz como donante de genes de resistencia a Fusarium asociado a bajo contenido de micotoxinas. Tesis Maestría: Genética Vegetal. Facultad de Cs. Agrarias. Universidad de Rosario (FCA, UNR), Santa Fe, Argentina / EEA INTA Pergamino, Buenos Aires, Argentina.
Ignjatović Micić, D., Ristić, D. Babić, V., Andjelković, V., Marković, K., Vančetović, J. (2013) Genetic assessment of maize landraces from former Yugoslavia. Genetika 45, Issue 2 (405-417). https://doi.org/10.2298/GENSR1302405I
Instituto Nacional de Tecnología Agropecuaria (INTA) (1972). Carta de suelos de la República Argentina.
Javed, R. M., Iqbal, S., Ullah, M.R., Khan, A., Iqbal, M.S., Ullah, M.U., Rehman, F. U., Khan, Saqib, M.S., Ali, S. (2021). Phenotypic and molecular divergence in maize (Zea mays L.) ecotypes. Pak. J. Agri. Sci. 58:1777-1787. https://doi.org/10.21162/PAKJAS/21.1469
Joshi, B., Rawat, J., Adhikari, B., & Pokhrel, R. (2020). SSR Markers Based Genetic Diversity in Nepalese Maize Landraces. SAARC Journal of Agriculture, 18(1), 23–37. https://doi.org/10.3329/sja.v18i1.48379
Kleinhofs, A., Kilian, A., Saghai Maroof, M. A., Biyashev, R. M., Hayes, P., Chen, F. Q., Lapitan, N., Fenwich, A., Blake, T. K., Kanazin, V., Ananiev, E., Dahleen, L., Kudrna, D., Bollinger, J., Knapp, S.J., Liu, B., Sorrells, M., Heun, M., Franckowiak, J. D., Hoffman, D., Skadsen, R., Steffenson, B. J. (1993) A molecular, isozyme and morphological map of the barley (Hordeum vulgare) genome. Theoretical and Applied Genetics 86:705-712. https://doi.org/10.1007/BF00222660
Labate, J. A., Lamkey, K. R., Mitchell, S. E., Kresovich, S., Sullivan, H., Smith, J. S. C. (2003) Molecular and historical aspects of Corn Belt Dent diversity. Crop Science 43:80.91. https://doi.org/10.2135/cropsci2003.8000
Lopes, C. A., Rodríguez, M. E., Querol, A., Bramardi, S. J., Caballero, A. C. (2006) Relationship between molecular and enological features of Patagonian wine yeasts: relevans in selection protocols. World Journal of Microbiology and Biotechnology 22:827-833. https://doi.org/10.1007/s11274-005-9110-4
López, C. G., Eyhérabide, G. H., Lorea, R. D., Delucchi, C., Percibaldi, N. M., Castelarin, J., Pedrol, H., Borrás, F. (2005) Selección de poblaciones locales de maíz como fuente de alelos favorables para el mejoramiento en un híbrido flint x dentado. Actas VIII Congreso Nacional de Maíz. Rosario. Pp: 358-360.
Lucchin, M., Barcaccia, G., Parrini, P. (2003) Characterization of a flint maize (Zea mays L. convar. Mays) Italian landrace: I. Morpho-phenological and agronomic traits. Genetic Resource and Crop Evolution 50:315-327. https://doi.org/10.1023/A:1023578207258
Luna, J. T., Safont Lis, J. (1978) El maíz en la Argentina: Vulnerabilidad y Recursos Genéticos.Ciencia e investigación. Tomo 3 – 4 – 5 y 6: 83 – 90.
Maize Data Bank www.agron.missouri.edu/Coop/SSR-Probes/SSR1.html.
Dias, P. M. B., Julier, B., Sampoux, J. P., Barre, P., Dall’Angol, M. (2008) Genetic diversity in red clover (Trifolium pretense L.) revealed by morphological and microsatellite (SSR) markers. Euphytica 160:189-205. http://dx.doi.org/10.1007/s10681-007-9534-z
Paz, N. M., Schlatter, A. R., Letis, G., Ferrer, M. (2005) Caracterización molecular de poblaciones locales de maíz. Actas VIII Congreso Nacional de Maíz. Rosario. Pp: 361-363.
Paz, N. M. (2009) Caracterización de la variabilidad genética en poblaciones locales de maíz mediante marcadores microsatélites. Tesis Maestría en Genética Vegetal; Área Mejoramiento Genético. UNR/INTA. Magister Scientiae, Mayo, 2009. 93p.
Pilling, D., Bélanger, J., Diulgheroff , S., Koskela, J., Leroy, G., Mair, G. and Hoffmann, I. (2020) Global status of genetic resources for food and agriculture: challenges and research needs : Global status of genetic resources for food and agriculture, Genetic Resources, 1(1), pp. 4–16. https://doi.org/10.46265/genresj.2020.1.4-16
Prevosti, A. (1974) La distancia genética entre poblaciones. Miscellanea Alcobé. Universidad de Barcelona, 109-118.
Presello, D. A., Ferrer, M., Solari, L., Céliz, A. (1996) Resistencia al virus del Mal de Río Cuarto en variedades locales argentinas de maíz. RIA, 27 (1): 19 a 26.
Presello, D.A., Iglesias, J., Botta, G., Reid, L. M., Lori, G. A., Eyhérabide, G. H. (2006) Stability of maize resistance to the ear rots caused by Fusarium graminearum and F. verticilloides in Argentinian and Canadian environments. Euphytica. 147: 403-407. https://doi.org/10.1007/s10681-005-9037-8
Reif, J. C., Melchinger, A. E., Xia, X. C., Warburton, M. L., Hoisington, D. A., Vasal, S. K., Srinivasan, G., Bohn, M., Frisch, M. (2003) Genetic distance based on simple sequence repeats and heterosis in tropical maize populations. Crop Science 43:1275- 1282. https://doi.org/10.2135/cropsci2003.1275
Rivas, J. G., Gutierrez, A. V., Defacio, R. A., Schimpf, J., Vicario, A. L., Hopp, H. E., Paniego, N. B. and Lía, V. V. (2022) Morphological and genetic diversity of maize landraces along an altitudinal gradient in the Southern Andes. PLoS ONE 17(12): e0271424. https://doi.org/10.1371/journal.pone.0271424.
Rohlf, F. J. (2002) NTSYSpc: Numerical Taxonomy System, ver. 2.1. Exeter Publishing, Ltd. Setauket. NY.
Salhuana, W., Pollak, L. M., Ferrer, M. E., Paratori, O., Vivo, G. (1998) Breeding Potential of Maize Accessions from Argentina, Chile, USA, and Uruguay. Crop Sci. 38:866-872. https://doi.org/10.2135/cropsci1998.0011183X003800030040x
Secretaría de Agricultura, Ganadería, Pesca y Alimentación (1997). Resolución 757/9: MAIZ: establécese un Reglamento Técnico de Identidad de Maíz Flint o Plata. 13 de octubre de 1997 https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-757-1997-46664/texto
Solari, L. R. (2007) IV Catálogo de Germoplasma de Maíz. Buenos Aires: INTA. 78p.:il. + CD-ROM. ISBN 978-987-521-293-0.
Sparks, A. 2018. “nasapower: A NASA POWER Global Meteorology, Surface Solar Energy and Climatology Data Client for R.” The Journal of Open Source Software, 3(30), 1035. https://doi.org/10.21105/joss.01035.
Tao, K., Li, Y., Hu, Y., Li, Y., Zhang, D., Li, C., He, G., Song, Y., Shi, Y., Li, Y., Wang, T., Lu, Y., Liu, X. (2023) Overexpression of ZmEXPA5 reduces anthesis-silking interval and increases grain yield under drought and well-watered conditions in maize. Mol Breeding 43, 84. https://doi.org/10.1007/s11032-023-01432-x
Torres-Morales, B., Rocandio-Rodríguez, M., Santacruz-Varela, A., Córdova-Téllez, L., Coutiño-Estrada, B., López Sánches, H. (2023) Genetic diversity characterization of maize populations using molecular markers. Italian Journal of Agronomy: 18 (3):2206. https://doi:10.4081/ija.2023.2206
Troyer, A. F., Openshaw, S. J., Knittle, K. H. (1988) Measurement of Genetic Diversity Among Popular Commercial Corn Hybrid Crop Sci. 28: 481-485. https://doi.org/10.2135/cropsci1988.0011183X002800030010x
Vigouroux, Y., Glaubitz, J. C., Matsuoka, Y., Goodman, M. M., Sánchez, J., Doebley, J. (2008) Population structure and genetic diversity of New World maize races assessed by DNA microsatellites. Am J Bot 95:1240–1253. doi: https://doi.org/10.3732/ajb.0800097
Warburton, M. L., Xianchun, X., Corssa, J., Franco, J., Melchinger, A. E., Frisch, M., Bohn, M., Hoisington, D. (2002) Genetic characterization of CIMMYT inbred maize lines and open pollinated populations using large scale fingerprinting methods. Crop Science 42:1832-1840. https://doi.org/10.2135/cropsci2002.1832
Wright, S. (1978) Evolution and the genetics populations. Vol. 4, Variability within and among natural populations. Univ. Chicago Press, Chicago.
Xiang, K., Yang, K. C., Pan, G. T., Reid, L. M., Li, W. T., Zhu, X., Zhang, Z. M. (2010) Genetic diversity and classification of maize landraces from China’s Sichuan Basin based on agronomic traits, quality traits, combining ability and SSR markers. Maydica 55(1):85-93.
Zuliani, P., Defacio, R., Lavalle, A., Bramardi, S. (2018). Comparación de técnicas de Análisis Multivariado mediante simulación para caracterización de recursos fitogenéticos en función de caracteres susceptibles a interacción genotipo-ambiente. Revista FAVE Sección Ciencias Agrarias, Universidad Nacional del Litoral. 17(1): 75-86. https://doi.org/10.14409/fa.v17i1