Solanum wild relative species indicate varying ecological resilience to climate change in Benin (West Africa)
Ahuéfa Mauricel Kégbéa,c, Rodrigue Idohoub,c,*, Birane Diengd, Gafarou Agoundec, Anthony Egerue,f, Kandioura Nobad, Achille Ephrem Assogbadjoa,c
a Laboratoire d’Ecologie Appliquée, Faculté des Sciences Agronomiques, Université d’Abomey-Calavi, 01 BP 526, Cotonou, Bénin
b Ecole de Gestion et de Production Végétale et Semencière, Université Nationale Agriculture, BP 43, Kétou, Bénin
c Laboratoire de Biomathématiques et d’Estimations Forestières, Faculté des Sciences Agronomiques, Université d’Abomey-Calavi, 04 BP 1525, Cotonou, Bénin
d Laboratory of Botany-Biodiversity, Faculty of Sciences and Techniques, Department of Plant Biology, Cheikh Anta Diop University, P.O Box 5005, Dakar, Fann, Senegal
e Department of Geography and Climatic Sciences, Makerere University, PO Box 7062, Kampala, Uganda
f Regional Universities Forum for Capacity Building in Agriculture (RUFORUM), P.O. Box 16811, Wandegeya, Kampala, Uganda
* Corresponding author: Rodrigue Idohou (rodrigidohou@gmail.com)
Abstract: Crop wild relatives are rich reservoirs of valuable genes for improving crop yields, but they have long been underestimated and neglected. Unfortunately, these resources are severely threatened in their natural habitats due to increasing stress caused by climate change and human disturbance. Recently, these wild species began receiving increasing attention for their effective inventory and sustainable conservation and use for the benefit of humanity. This study investigated the current distribution and forecasted the potential future climate change impact on ten Solanum wild relative species in Benin, assessed the effectiveness of protected areas in maintaining viable populations, and evaluated their conservation status using the International Union for Conservation of Nature Categories and Criteria. We used species distribution models under two socioeconomic pathways SSP370 and SSP585 projecting species ranges for the 2055 and 2085-time horizons. The models demonstrated high accuracy with an average value of the Area Under the Curve and True Skill Statistic of 0.89 and 0.74, respectively. The most suitable areas were located in the Sudano-Guinean and Guineo-Congolian zones of Benin. Furthermore, a significant proportion of these suitable areas is projected to become unsuitable for most wild Solanum species. Surprisingly, most of the identified hotspots were poorly represented within the existing protected area network, which appears insufficient to provide long-term refugia for the species. Nevertheless, new suitable areas were identified outside the current protected zones. Coordinated efforts are urgently needed to sustainably manage the populations of target species to enhance their future persistence in Benin.
Keywords: Crop wild relatives, climate change, protected area networks, Solanum, species distribution modelling
Introduction
Crop wild relatives (CWR) are wild plant species closely related to cultivated crops and possess valuable traits that can contribute to crop improvement and breeding efforts. Found in natural habitats, these wild species have co-evolved with domesticated crops over millennia (Maxted et al, 2006; Maxted et al, 2012). The genetic diversity present in CWR is crucial for enhancing the resilience and adaptability of agricultural systems, particularly in response to changing environmental conditions and the emergence of new pests and diseases (Maxted and Magos Brehm, 2023). However, habitat loss, climate change, and other human activities threaten the survival of these species (Idohou et al, 2025), limiting their potential contribution to food security, nutrition and human health. Despite these challenges, CWR have received and continue to receive increasing interest from scientists worldwide (Pilling et al, 2020).
The Solanaceae family is highly diverse, comprising over 2,000 species across approximately 90 genera, including major crops, their wild relatives, and a wide range of perennial and herbaceous annual species (Samuels, 2015; Gebhardt, 2016). Many species provide a wide range of goods and services (Gebhardt, 2016), and are used for food, medicinal and ornamental purposes (Samuels, 2015). In Benin, wild progenitors of Solanum are distributed throughout the country but are more abundant in the Sudano-Guinean and the Guineo-Congolian zones (Akoègninou et al, 2006). In fact, wild Solanum species also play an important role in the diet of the local population (Sarma and Sarma, 2011). Several of such species are directly consumed as wild foods rich in micronutrients, fibre and antioxidants. They diversify diets and enhance local food security, especially in rural and marginalized communities.
Fruits and leaves are used for soups or sauces and have been shown to successfully contribute to improving human health (Sarma and Sarma, 2011; Okokon et al, 2017). For instance, Solanum species have been reported to be effective in the treatment of various diseases, including malaria, stomach aches, asthma and diabetes (Sarma and Sarma, 2011). Despite their importance, wild relatives of cultivated Solanum vegetables are currently facing severe threats (Syfert et al, 2016) in their natural habitats due to habitat loss and conversion primarily from agriculture and grazing (Idohou et al, 2017; Lala et al, 2018) and more critically, climate change. Climate change impacts all aspects of agriculture, which remains a key economic sector and a cornerstone of food security and nutrition in Africa. According to the Intergovernmental Panel on Climate Change (IPCC), changes in temperature, precipitation patterns, and the frequency of extreme weather events have been observed since the 1950s (Masson-Delmotte et al, 2022). Climate change is now considered one of the major threats to biodiversity and food production, causing widespread disruptions to human systems and indigenous livelihoods, particularly in West Africa (IPCC, 2021). As conservation efforts increasingly adopt a ‘biodiversity for livelihoods’ approach, accurate information on plant distributions and ecosystem shifts under environmental change is urgently needed.
In the face of this global change, it became urgent to assess the distribution patterns of Solanum wild relatives under climate change. Indeed, many wild relatives of Solanum are found in both agricultural and protected areas (PA), which are currently experiencing high levels of disturbance (Steffen et al, 2015). Given the current distribution of these species and the potential impact of climate change in many ecosystems across Africa, we hypothesized that such environmental changes would negatively affect the future distribution ranges of wild relatives of cultivated Solanum leafy vegetables in Benin. We also assumed that abiotic factors, namely rainfall, temperature and soil, are the primary drivers influencing both the current and future distribution of these species.
Ecological niche models (ENM), also known as species distribution models (SDM) are powerful tools widely used to predict the species’ abiotic niches and forecast their future distributions under climate change scenarios (Feng et al, 2020). Various methods have been applied to assess species distributions (Hijmans and Elith, 2017); however, the choice of the method largely depends on the aim of the study and the availability of data. Maximum Entropy Modelling (MaxEnt) (Phillips et al, 2006) is among the most frequently used SDM algorithms. It requires only presence data as inputs and estimates species’ relative occurrence rates (Yackulic et al, 2013) by minimizing the relative entropy between the probability distributions of species presence and the background environment. In Benin, several researchers have explored the effectiveness of MaxEnt in predicting the potential impacts of climate change on species distribution (Daï et al, 2023; Hounsou-Dindin et al, 2023). However, no studies have specifically focused on the wild relatives of cultivated Solanum leafy vegetables to assess their adaptive responses to predicted climate variations. Furthermore, the assessment of PA effectiveness in conserving genetic resources must be included in such studies, given their crucial role in safeguarding biodiversity from human disturbances (Stolton et al, 2006). In addition, PA serve as natural refuges for many wild plants, although these species have long been overlooked within these conservation frameworks (Vargas et al, 2004; Burgess et al, 2005). Given the limited occurrence of wild Solanum species in PA, we hypothesized that these ecosystems are unlikely to serve as hotspots for them.
This study examined the potential impact of climate change on wild relatives of cultivated leafy Solanum vegetables in Benin to inform appropriate conservation strategies. Specifically, it aimed to (1) identify the abiotic factors influencing the distribution of the species and their hotspots, (2) evaluate both current and future shifts in the species distribution, (3) determine the conservation status of the target Solanum species in Benin based on the International Union for Conservation of Nature (IUCN) Red List Categories and Criteria and (4) assess the effectiveness of PA in conserving species distributions.
The research questions for this study are as follows: Which factors influence the geographical distribution patterns of Solanum wild relatives? What conservation strategies could mitigate these impacts and enhance the resilience of the species to climate change?
Materials and methods
Study area
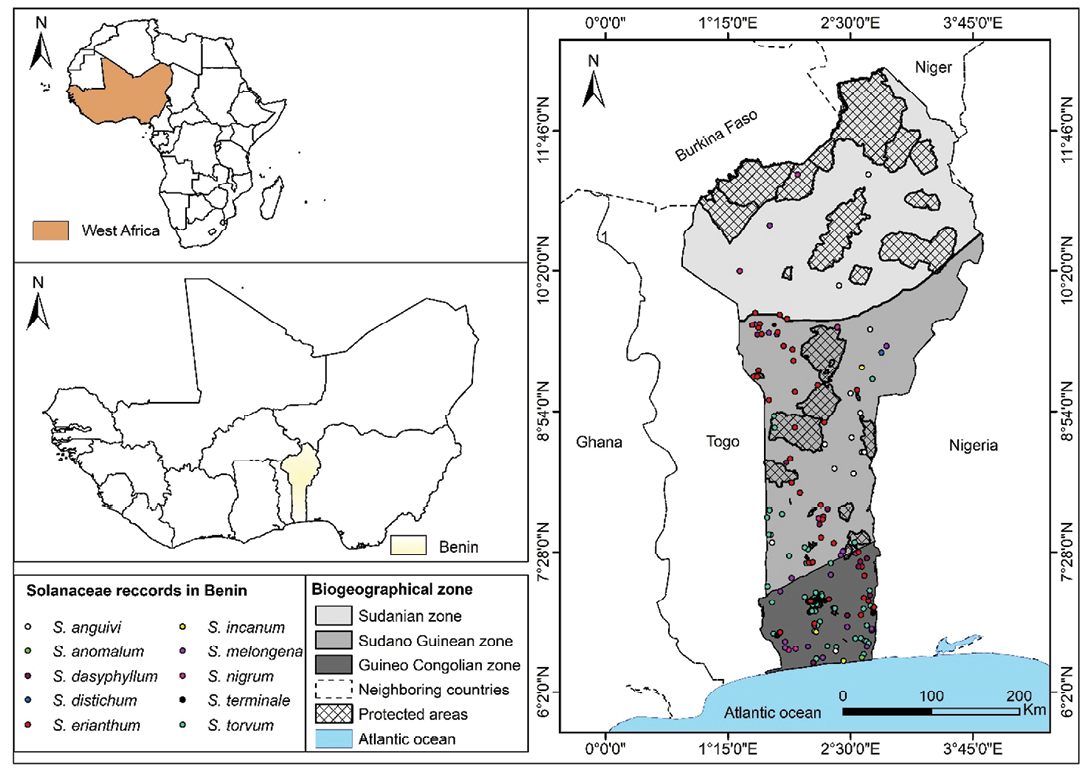
The study was carried out in the Republic of Benin located in West Africa between 6°0’ and 12°50’N, and 1°0’ and 3°40’E (Figure 1). Three biogeographical zones are distinguishable, namely Sudanian, Sudano-Guinean, and Guineo-Congolian (Adomou, 2005; Figure 1). In the Guineo-Congolian, the mean annual temperature is generally 25–29°C. The relative humidity is 69–97% with a bimodal annual rainfall of 1,200mm. The land cover types are mainly fallow land and agricultural fields with the highest human population density. As for the Sudanian zone, it is the driest in the country. However, the rainfall is also unimodal, sometimes reaching 1,000mm per year (Adomou, 2005). Temperatures range from 24 to 31 °C with a relative humidity between 18 and 99%. The vegetation is characterized by dry forests, riparian forests, tree, shrub and herb savannahs and woodlands. The transition zone of the Sudano-Guinean region is dominated by bimodal rainfall with a tendency towards unimodality varying between 900 and 1,110mm per year (Adomou, 2005). The annual temperature ranges from 25 to 29°C and the relative humidity varies from 31 to 98%. Here, the vegetation is characterized by riparian forests, woodlands and dry forests. The flora of Benin is estimated to comprise 2,807 plant species, grouped into 1,129 genera and 185 families (Akoègninou et al, 2006). The species-rich families are Leguminosae (14.8%), Poaceae (9.3%), Rubiaceae and Cyperaceae (5% each), Asteraceae (4.6%) and Euphorbiaceae (4.3%) (Akoègninou et al, 2006; Adomou, 2005). Agriculture contributes about 28,04% to the gross national product, and the main crops cultivated in Benin are cereals, legumes, tubers, vegetables and industrial crops (MAEP, 2020).
Solanum species selection
A previous study by Idohou et al (2013) generated a list of priority CWR for Benin for conservation. The list was used to extract the existing list of Solanum wild relatives’ species in Benin. The species names were cross-checked with those existing in the Analytic flora of Benin (Akoègninou et al, 2006). The species considered in this study were: Solanum anguivi Herb.Lamb. ex Dunal, S. anomalum Thonn., S. dasyphyllum Schumach. & Thonn., S. distichum Schumach. & Thonn., S. erianthum D.Don, S. incanum Ruiz & Pav., wild forms of S. melongena Ruiz & Pav., S. nigrum Vell., S. terminale Forssk., and S. torvum Buch.-Ham. ex Wall. The description, distribution, ecology, uses, threats and conservation status of each species are summarized in Supplemental Table 1.
Occurrence data
The occurrence records for the ten species were initially downloaded from open-access platforms such as the Global Biodiversity Information Facility (www.gbif.org) and RAINBIO (http://rs.tdwg.org/dwc/terms/). Records with the same coordinates and those without identification information were removed with ENMTools (www.ENMTools.com) (Warren et al, 2010). To minimize temporal bias and ensure compatibility with the climate datasets, records prior to 2000 were excluded (Idohou et al, 2017). Additionally, further occurrence data were collected during a field expedition conducted between 2019 and 2021 across the Sudano-Guinean and Guineo-Congolian zones. These records encompass a diverse array of ecosystems including farms, fallows and semi-deciduous forests. To reduce spatial autocorrelation among occurrence records, we used the occurrence rarefy tool in ArcGIS v.10.8 Spatial Analyst tool (Brown, 2014) and retained only one record per 1×1km grid cell. Initially, 1,175 raw occurrences were compiled; after filtering for uniqueness and accuracy, 693 records remained, of which 636 were ultimately used in ecological niche modelling (Supplemental Table 2). The minimum number of georeferenced occurrence records was 20, aligning with the recommendations of Wisz et al (2008), who advised 25 records when performing SDM for African species.
Table 1. Number of records of wild Solanum species and their sources. GBIF, Global Biodiversity Information Facility; RAINBIO, Mega-database of tropical African vascular plants distributions.
|
Species |
Field |
GBIF |
RAINBIO |
Raw data |
Used data |
|
Solanum anguivi Herb.Lamb. ex Dunal |
30 |
98 |
0 |
128 |
20 |
|
Solanum anomalum Thonn. |
10 |
22 |
9 |
41 |
25 |
|
Solanum dasyphyllum Schumach. & Thonn. |
14 |
101 |
14 |
129 |
43 |
|
Solanum distichum Schumach. & Thonn. |
52 |
52 |
11 |
115 |
22 |
|
Solanum erianthum D.Don |
28 |
50 |
9 |
87 |
60 |
|
Solanum incanum Ruiz & Pav. |
0 |
90 |
27 |
117 |
15 |
|
Solanum melongena Ruiz & Pav. |
3 |
114 |
14 |
131 |
102 |
|
Solanum nigrum Vell. |
30 |
106 |
6 |
142 |
115 |
|
Solanum terminale Forssk. |
0 |
14 |
21 |
35 |
31 |
|
Solanum torvum Buch.-Ham. ex Wall. |
45 |
205 |
0 |
250 |
203 |
|
Total |
212 |
852 |
111 |
1,175 |
636 |
Environmental data
Current and future climatic data at 30 arcsec spatial resolution (~1km at the equator) were obtained from the Chelsa website (www.chelsa-climate.org) (Parviainen et al, 2008). The datasets included 19 bioclimatic variables (Supplemental Table 3) related to temperature and rainfall (Karger et al, 2017) and represented a baseline condition (1979 to 2013) along with two future periods: 2055 (2041–2070) and 2085 (2071–2100). Future climate conditions were projected using the IPSL-CM6A-LR climate model (Boucher et al, 2020), under two shared socioeconomic pathways (SSP): the middle-of-the-road scenario (SSP3-7.0) and the worst-case scenario (SSP5-8.5). These scenarios predict conditions likely for Africa by 2055 and 2085 (Williams and Jackson, 2007). SSP3-7.0 predicts an additional radiative forcing of 7W/m2 by 2100 while SSP5-8.5 forecasts an additional radiative forcing of 8.5W/m2 by 2100. The two scenarios were used to represent the moderate and the worst-case impacts of climate change for wild Solanum species in Benin. In addition, edaphic variables were downloaded from the Africa Soil Profiles Database (https://www.isric.org) and processed for Benin. The selected variables are summarized in Supplemental Table 3. Elevation data were obtained from the WorldClim database (www.worldclim.org). All these variables had a spatial resolution of 1km and were merged using the raster package in R 4.0.3 (R Core Team, 2023). To determine the environmental factors influencing the current distribution of each taxon, we followed a 3-step procedure. First, we excluded bio8, bio9, bio18, and bio19 because these variables frequently exhibited discontinuities between neighboring pixels across the African continent (Montoya-Jiménez et al, 2022). Second, we performed a Pearson correlation test using the usdm package (Naimi, 2017) to select variables with low correlation, retaining those with an absolute correlation coefficient (|r|) below 0.7 (Supplemental Table 4). Third, we conducted a jackknife test using the SDMtune package (Vignali et al, 2020) to identify variables that contributed significantly to the models. The final set of environmental factors used in modelling the ecological niche of each species was the least correlated, which made a substantial contribution to the models, and was ecologically relevant.
Modelling technique
Forty SDM were built per species (4 methods x 2 replication methods x 5 replicates). Four machine learning (ML) models available in the sdm package version 1.2-46 (Naimi and Araujo, 2016) were used: boosted regression trees (BRT) (Friedman et al, 2000), random forests (RF) (Breiman, 2001), support vector machines (Meyer and Wien, 2001) and MaxEnt (Phillips et al, 2006; Phillips et al, 2017). This ensemble modelling approach enhances model accuracy and robustness compared to single-algorithm methods (Ahmad et al, 2020). Due to the lack of true absence data, we generated 104 pseudo-absences as recommended (Barbet-Massin et al, 2012) using the sdm package. The dataset was split into 70% for model training and 30% for testing, and model fitting was expedited using parallel processing across four ‘n-cores’ (Naimi and Araujo, 2016). Model performance was evaluated using 5-fold cross-validation and bootstrapping replication methods. An ensemble function was applied to integrate predictions from all four machine learning models. Performance metrics included the area under the receiver operating characteristic (ROC) curve (AUC) and the true skill statistic (TSS). The AUC indicates the probability that the predictive power of a model is better than random prediction (AUC = 0.5) (Ramírez-Villegas et al, 2010; Castañeda-Álvarez et al, 2015); a model with an AUC value close to 1 (AUC ≥ 0.75) is considered to have a good fit. The TSS is a measure of the ability of the model to detect true presence (sensitivity) and true absence (specificity). It is expressed as sensitivity plus specificity -1, with TSS > 0.5 indicating a good predictive power (Zhang et al, 2015). Finally, suitability layers representing the current and future distribution of each species were exported in binary raster TIFF format using the average TSS value from the five replications, where a value of 1 denotes suitable habitat and 0 denotes unsuitable habitat.
Suitability habitat mapping, dynamic of the suitable areas, species richness and gap analysis
The output raster representing the habitat suitability of each species (i.e. suitable and unsuitable habitats) was imported into ArcGIS 10.8, and the current and future suitable areas were then mapped.
Habitat dynamics were quantified using the Spatial Analyst tool in GIS by counting the total number of pixels corresponding to the current and future distribution. The Rate of Change Index (RCI) was calculated using the following formula (Coulibaly et al, 2021):
FA corresponds to the future area (e.g. suitable) of a species in the target horizon under scenario i (here horizon = 2055 and i = SSP3-7.0 and SSP5-8.5); CA corresponds to the current distribution area (e.g. suitable); ∆ is the percentage of area gain (∆ > 0) or lost (∆ < 0) and stable ∆ = 0. A pattern of change was qualified as minor when values varied between (0–5%), (6–15%) indicated moderate decrease and the upper 21% major decrease.
The dynamics of species richness over time were evaluated by summarizing the binary (1 = presence, 0 = absence) layers for the present and future distributions of the ten Solanum species. In this context, species richness referred to the number of Solanum species found in 1km2 area, based on the resolution of environmental variables used in the models. Richness levels were then classified into five different categories based on the number of Solanaceae species modelled: high (8–10), moderate (5–7), low (2–4), very low (1) and no species (0).
Gap analysis was conducted by overlaying the shapefile of Benin's PA network (World Database on Protected Areas, UNEP-WCMC 2023) with the habitat suitability and species richness maps. This analysis aimed to evaluate the effectiveness in covering suitable habitats and to identify potential priority areas for future conservation efforts.
Conservation status assessment of the species
In this study, we assessed the conservation status of ten Solanum species in Benin using projections from SDM under future climate scenarios. Although many studies traditionally apply IUCN Criterion B by estimating the extent of occurrence (EOO) and area of occupancy (AOO) (e.g. Dassou et al, 2024), criterion A3(c) is more appropriate for SDM as it considers the expected reduction in population size inferred from the projected decline in habitat suitability. Criterion A3(c) relates to a reduction in EOO, AOO or habitat quality up to a maximum of 100 years (IUCN, 2024). According to IUCN guidelines, a species is classified as Extinct (EX) if it is projected to lose 100% of its suitable area, Critically Endangered (CR) with ≥ 80% reduction, Endangered (EN) with ≥ 50% and < 80%, Vulnerable (VU) with ≥ 30% and < 50%, Near Threatened (NT) with < 30%, and Least Concern (LC) if its suitable area is stable or increasing. The methodology for calculating the percentage change in the suitable area and for assigning IUCN categories was detailed in the previous section. In Benin, threats to CWR, including Solanum species, fall into three major categories: (1) agricultural expansion and urbanization, leading to habitat loss, (2) overharvesting, resulting in population decline, and (3) invasive species and climate change, also contributing to population decline (Idohou et al, 2013). Among these, agricultural encroachment has been identified as the most significant threat to Solanum species nationwide, severely affecting their suitable habitats. Consequently, the preliminary conservation status of each species was determined by combining the predicted reductions in suitable area from SDM with an understanding of ongoing threats.
Results
Variable contribution, model validation and performance evaluation
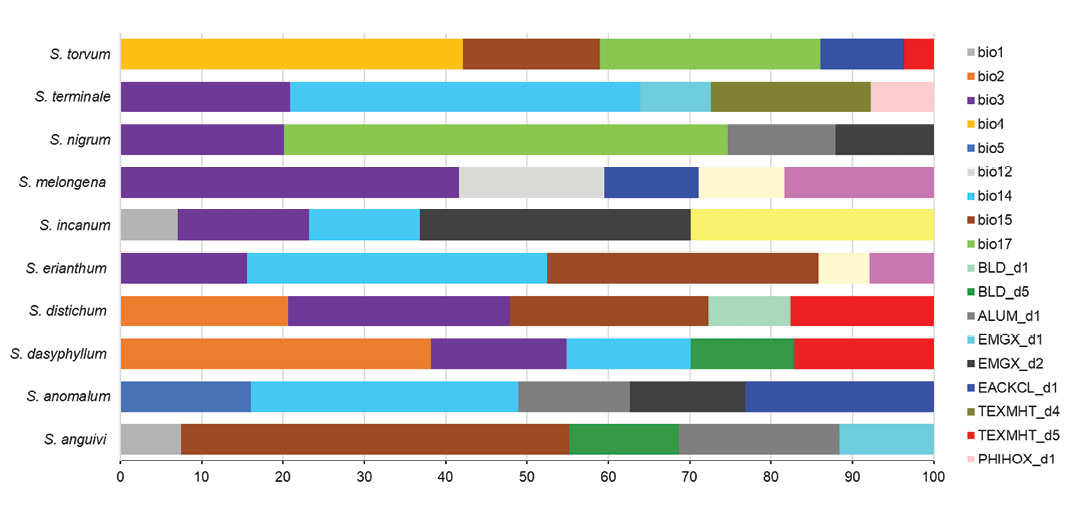
A total of 21 non-correlated variables (Figure 2) were identified as important in determining the modelled distribution of the ten Solanum species. Among these, temperature- and precipitation-related variables had a significantly greater influence on the SDM compared to soil and elevation variables. Individually, isothermality (bio3) emerged as the most influential variable shaping the distribution of the Solanum species. However, precipitation of the driest quarter (bio17) had the highest individual contribution, particularly for S. nigrum with contributions of 27.16% and 24.23%, respectively. S. melongena showed the highest dependence on bio3 (42%), followed by S. distichum (27.27%), S. terminale (20.87%), S. nigrum (20.11%), and S. dasyphyllum, S. erianthum and S. incanum, each with contributions around 16.5%. Annual temperature (bio1) moderately influenced the distribution of S. anguivii and S. incanum, each with a contribution of around 7%. The mean diurnal range (bio2) was a major predictor for S. dasyphyllum (38.17%) and S. distichum (20.66%). Temperature seasonality (bio4) and maximum temperature of the warmest month (bio5) were key variables for S. torvum (42.11%) and S. anomalum (16.04%), respectively. Precipitation seasonality of (bio15) was the most influential factor for S. anguivii (47.74%), followed by moderate to low contributions for S. erianthum (33.37%), S. distichum (24.39%) and S. torvum (16.84%). S. terminale strongly depended on precipitation of the driest month (43.10%) (bio14) while S. anomalum and S. erianthum also responded significantly (33.5% and 37.5%, respectively). For S. incanum, however, bio14 had a relatively low contribution (13.66%). Overall, soils characteristics showed a weak contribution for all the species. Among those, exchangeable acidity in subsoil (EACKCL_d1) and exchangeable magnesium (EMGX_d2) had some effect on S. incanum, S. anomalum, S. nigrum, S. torvum and S. melongena. Bulk density in topsoil (BLD_d1) contributed 10.05% for S. distichum, while subsoil bulk density (BLD_d5) contributed approximately 13% for both S. anguivi and S. dasyphyllum. Subsoil aluminium concentration (ALUM3S_d1) had contributions ranging from 13% to 18% for S. anguivi, S. anomalum and S. nigrum. Soil texture class at 45cm depth weakly affected S. terminale (19.64%), while deeper texture layers (TEXMHT_d5) impacted S. dasyphyllum (17.11%), S. distichum (17.64%) and S. torvum (3.68%).
Silt fraction (SLTPPT_d1) influenced S. melongena (18.7%) and S. erianthum (7.6%). Soil pH in the topsoil (PHIHOX_d1) slightly influenced S. terminale (7.73%), and subsoil pH (PHIHOX_d6) impacted S. erianthum (6.27%) and S. melongena (10.65%).
Elevation (DEM) was a significant predictor only for S. incanum, contributing 29.86% to its distribution model.
Figure 2. Contribution (%) of the variables to the models. The models were built using an ensemble of four algorithms boosted regression trees (BRT), random forests (RF), support vector machines (SVM), and MaxEnt with five replications each. Variable contributions were averaged across algorithms and replications to obtain final estimates.
The results showed an average AUC value of 0.89 across the ten Solanum species (Table 2), indicating that SDM performed well in predicting their potential distributions. Similarly, the average TSS value was 0.74, reflecting a strong agreement between observed and predicted occurrences.
Table 2. Model performance based on the two metrics. AUC, Area under the curve; TSS, True skill statistic.
|
Species |
AUC |
TSS |
|
0.9 |
0.8 |
|
|
S. anomalum |
0.9 |
0.81 |
|
S. dasyphyllum |
0.83 |
0.63 |
|
S. distichum |
0.83 |
0.69 |
|
0.96 |
0.85 |
|
|
S. incanum |
0.8 |
0.5 |
|
S. melongena |
0.87 |
0.69 |
|
S. nigrum |
0.92 |
0.78 |
|
0.91 |
0.82 |
|
|
S. torvum |
0.94 |
0.81 |
Geographic distribution patterns of the species under current and future climate conditions
The categorization of habitats under current conditions showed a diverse range of suitable areas for all species except for S. anomalum (Figure 3). The primarily suitable habitats for the studied species were predominantly located in the Sudano-Guinean and Guineo-Congolian zones. In contrast, a significant portion of the coastal region was unsuitable for some species, notably S. anguivii and S. incanum. For S. anomalum, suitable areas were limited and mostly concentrated along the eastern coast of the Guineo-Congolian zone. A similar distribution pattern was observed for S. dasyphyllum, although this also showed a broader extent of suitable habitat extending into the Sudanian zone.
Under future climate scenarios (SSP3-7.0 and SSP5-8.5) for the horizons 2055 and 2085, a general decline in suitable habitats was projected for many species. However, S. melongena and S. dasyphyllum showed a clear expansion of suitable habitats. The suitable areas for S. distichum, S. nigrum and S. torvum remained relatively stable across all scenarios and time horizons. In contrast, S. anomalum, S. incanum and S. terminale were projected to lose the suitable habitats by 2055. For S. incanum, the distribution remains narrow and relatively stable in the future compared to its initial distribution. S. erianthum showed a decline in suitability, with its distribution shifting southwards into the Guineo-Congolian zone – this spatial trend was consistent across both horizons and scenarios. When overlaying habitat suitability maps with the PA network, varied patterns emerged. In the Sudanian zone, parts of the suitable habitats for some species overlapped with existing PA except for S. melongena and S. dasyphyllum, which were largely unprotected. Under future climatic conditions, some PA emerged as important conservation zones. For instance, the classified forests of Kétou and Dogo are projected to harbour significant hotspots of S. terminale. Similarly, the Lama Forest appears to be a potential refuge for S. incanum.
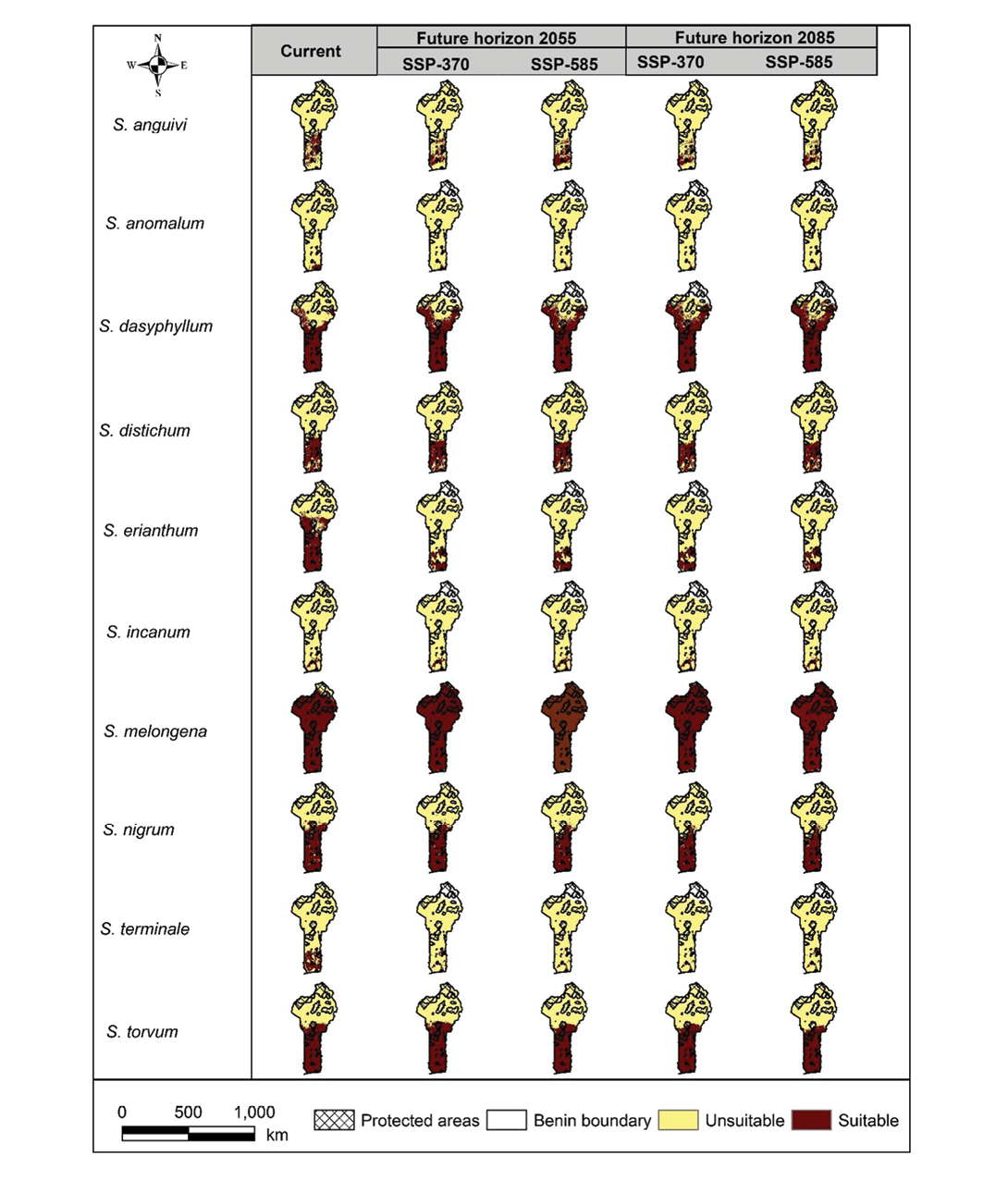
Dynamics of suitable habitats for Solanum species across Benin
The projected distribution of wild relatives of cultivated leafy vegetables of Solanum species, indicated that, under both present and future scenarios, the extent of suitable habitats will decrease for most species (Supplemental Table 5). Overall, eight out of ten species are expected to experience a reduction in suitable areas, while only two are projected to see an expansion.
Species such as Solanum anguivii, S. anomalum, S. distichum, S. erianthum, S. incanum, S. nigrum, S. terminale and S. torvum are projected to lose suitable habitats. Under current conditions, S. torvum occupies a suitable area of 4,560.47km². By 2055, this area is projected to increase by 7.06% under the SSP3-7.0 scenario but to decrease by up to 8.41% under SSP5-8.5. Other species, such as S. distichum and S. incanum, are predicted to experience relatively minor reductions in habitat suitability, with losses not exceeding 21%. The current suitable area of S. distichum is estimated at 2,601.14km², with projected reductions ranging from 11.09% to 20.49%, the largest occurring under SSP3-7.0 by 2085 (Supplemental Table 5). For S. incanum, the current suitable area is 743.28km², with projected losses of 17.74% under SSP3-7.0 and 14.06% under SSP5-8.5 by 2055. By 2085, this downward trend is expected to continue, with projected reductions of 14.06% and 14.06% under SSP3-7.0 and SSP5-8.5, respectively.
Moderate habitat losses, i.e. not exceeding 45%, were observed only for S. anguivii and S. nigrum. The current suitable areas for S. anguivii and S. nigrum were estimated at 1,583.58km² and 4,288.29km², respectively. However, the projected trends in habitat suitability are not stable for either species. For S. anguivii, a minor reduction of 15.09% is projected under the SSP5-8.5 scenario by 2055, whereas a more substantial reduction of 40.88% is expected under the same scenario by 2085. Similarly, under SSP3-7.0, reductions of 26.75% and 38.80% are projected for 2055 and 2085, respectively. S. nigrum exhibits the same pattern, with relatively minor reductions of 7.17% and 16.47% under SSP3-7.0 for 2055-time horizon and SSP5-8.5 time horizon, respectively, increasing to 21.91% under SSP3-7.0-2085 and 27.33% under SSP5-8.5-2085.
Species with major loss included S. anomalum, S. erianthum, and S. terminale, each experiencing projected reductions in suitable habitat exceeding 60% under all future scenarios (Supplemental Table 5). For instance, S. anomalum, which currently occupies 282.00km², is expected to lose up to 64.91% of its suitable area by 2085 under SSP5-8.5. Similarly, S. erianthum, with a current suitable area of 4,971.81km², is projected to decline by as much as 72.53% by 2055 under SSP3-7.0. S. terminale, currently distributed across 1381.48km², faces reductions up to 86.37% under the same scenario by 2085. These sharp contractions indicate a significant risk of habitat-driven population decline and potential local extirpation without targeted conservation efforts. In contrast, S. dasyphyllum and S. melongena are projected to expand their suitable habitats under all climate scenarios (Supplemental Table 5). S. dasyphyllum, currently found in 5,314.32km², may gain up to 57.15% by 2085 under SSP5-8.5, while S. melongena, with a current area of 10,717.61km², is projected to expand by 6.13% under the same scenario. Despite these positive trends, their future distributions remain susceptible to uncertainties inherent in climate projections, highlighting the need for continued monitoring. These contrasting patterns underscore the importance of adopting species-specific strategies for effective conservation planning in the face of climate change.
Dynamic of suitable areas of Solanum species within the protected areas network
Supplemental Table 6 presents the dynamics of suitable areas for Solanum species within the protected areas (PA) network. Overall, two main trends were observed, mirroring those at the national level. Eight out of ten species (S. anguivi, S. anomalum, S. distichum, S. erianthum, S. incanum, S. nigrum, S. terminale, and S. torvum) are projected to lose suitable area within PA, while only S. dasyphyllum and S. melongena are expected to gain.
S. anguivi currently occupies 1,790.92km² within PA and is projected to decline by up to 58.89% under SSP3-7.0 by 2085, reducing its extent to 736.27km². S. anomalum, with a current extent of 47.26km², shows consistent losses of 63.48% across all future scenarios, resulting in a stable yet severely reduced extent of 17.26km². S. incanum presently covers 3,158.83km² and is projected to decline by roughly 50% across all scenarios, reaching 1,561.9km² under SSP5-8.5 by 2085. S. torvum has a current distribution of 964.46km² and is projected to undergo losses under all scenarios, with the most severe reduction (62.66%) under SSP3-7.0 by 2085, dropping to 360.16km². While it displays a negligible gain of 0.55% in 2055 under SSP3-7.0, this is not sustained in later scenarios.
Moderate losses were observed for S. distichum, currently distributed over 3,472.39km². The most substantial reduction is projected under SSP5-8.5 by 2085, where the extent declines to 1,077.04km², accounting for a 68.98% reduction. S. nigrum shows contrasting dynamics, with a projected gain of 36.83% under SSP5-8.5 by 2055, but this is followed by a sharp decline (62.73%) under SSP5-8.5 by 2085, reducing its suitable area to 2,816.51km². S. terminale follows a similar trajectory, dropping from 7,557.58km² to 2,816.51km² under SSP5-8.5 by 2085 (62.73%). S. erianthum faces the most severe reductions, shrinking from 7,993.3km² to just 1,187.15km² by 2055 under SSP3-7.0 (85.15%) and showing similarly drastic losses under all scenarios.
By contrast, S. dasyphyllum and S. melongena are projected to expand within the PA network. S. dasyphyllum currently occupies 9,668.47km² and may increase its extent by up to 78.65% (17,272.45km²) by 2085 under SSP5-8.5. S. melongena, with the largest current extent (31,355.97km²), shows consistent expansion across all scenarios, peaking at 18.51% (37,159.88km²) under SSP5-8.5 by 2085. These contrasting dynamics emphasize the need for targeted conservation planning tailored to each species’ future trajectory within the protected areas network.
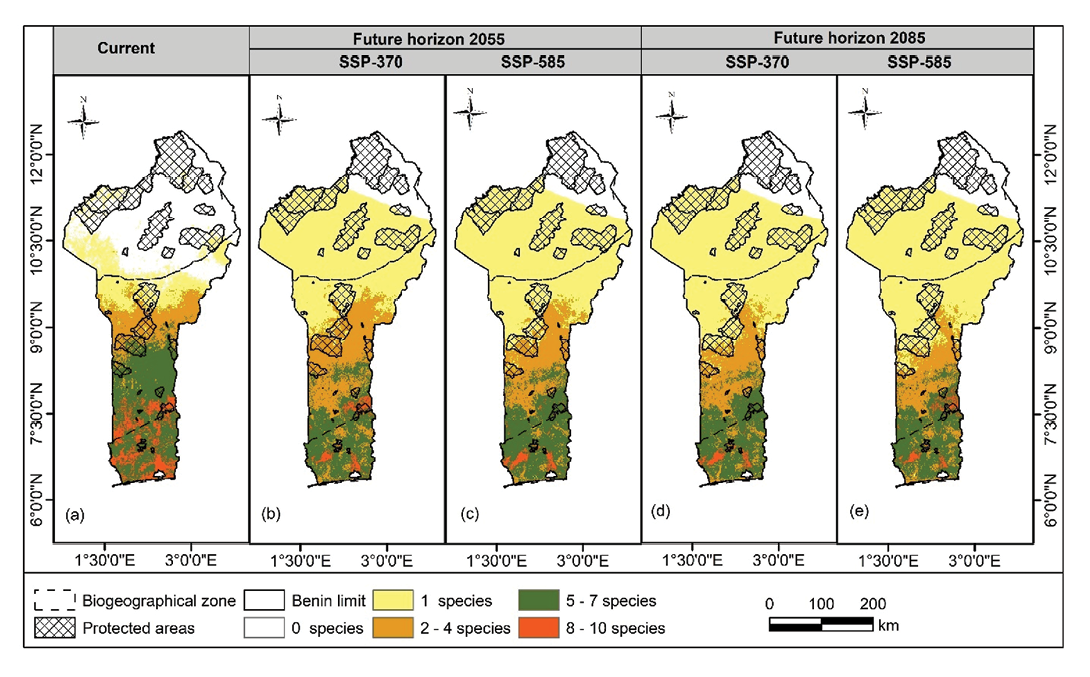
Solanum richness dynamic accounted for current and future distribution
Under current conditions, SDM indicate a decline in Solanum species richness towards the southern regions (Figure 4). The highest richness category comprising eight to ten species was predominantly concentrated in the Guineo-Congolian and Sudano-Guinean zones. This richness class currently covers an estimated 681.11km2 (5.94% of the total area). However, projections under future climate scenarios suggest a reduction of approximately 4.5% in this richness category (Supplemental Table 7). Moderate richness levels (5–7 species) were observed in the Ouémé-Boukou and Agoua PA, which are located in the Guineo-Congolian and Sudano-Guinean zones, respectively (Figure 4). Compared to the current condition, the future richness of the wild Solanum species is likely to decrease southwards (Figure 4). The current distribution area of Solanum species richness was estimated at 2,374.49km2 (20.69%) with a projected reduction ranging from 3.18 to 5.34% under future climate scenarios. However, our findings indicated an increase in the species richness (2–4 species class) in the Sudanian zone. When we considered the richness class 2–4, an expansion towards the Guineo-Congolian and Sudano-Guinean zones could occur. This class occupied 11.28% (1,294.75km2) of the total area (Supplemental Table 7). The increase in area ranged from 3.56–10.54% with the lowest value under SSP3-7.0 by 2055 and the highest value at SSP5-8.5 in 2085. As for the richness class (1 species), it occupied 16.39% (1,880.91km2). The increase ranged from 10.89–25.25% with the highest percentage under SSP5-85 in 2085 in terms of PA. The pattern of species richness shows that higher richness classes (5–7 and 8–10 species) are generally located outside the PA network. However, notable exceptions include the Kétou and Dogo PA, situated in the transition zones between the Sudano-Guinean and Guineo-Congolian regions. Under current conditions, richness classes with 0 and 1 species together account for approximately 66% of the richness within the PA network (Supplemental Table 8). Meanwhile, the 5–7 and 8–10 species richness classes make up only 11.37% of the total PA coverage.
The conservation status assessment of the Solanum genus
The conservation status of the ten Solanum species in Benin was based on the IUCN criteria. The results revealed varying levels of climate-induced vulnerability overall. One species was classified as CR, two as EN, three as VU, two as LC and two as NT. This was based on their major threats and distribution range (Supplemental Table 9), as well as the projected percentage changes in suitable habitat under the SSP3-7.0 and SSP5-8.5 scenarios for 2055 and 2085 (Supplemental Table 10).
S. terminale is the most severely affected species, with a projected habitat loss of over 70% under all future scenarios, reaching 85.86% under the SSP5-8.5 scenario by 2055. Coupled with its restricted distribution and high exposure to anthropogenic pressures, this supports its classification as CR. Similarly, S. erianthum and S. anomalum show consistent declines of between -53.06% and -69.42%, which justifies their classification as EN given their sensitivity to fire and habitat degradation. At the next level of severity, S. incanum, S. distichum and S. anguivi experience a moderate decline in suitable habitat, ranging from -14.10% to -40.88%. Despite having broader ecological amplitudes, these species face significant local threats, such as overgrazing, soil disturbance and agricultural encroachment. This warrants their classification as VU.
By contrast, S. dasyphyllum and S. melongena are projected to remain stable or even increase in number under future climate conditions, with changes ranging from -3.95% to +57.15%. Their wide ecological tolerance and lower exposure to critical threats mean they are designated as LC. However, ongoing monitoring is advised to detect any future shifts in vulnerability.
Finally, S. nigrum and S. torvum exhibit variable responses across scenarios, with projected changes ranging from a decline of 27.33% to an increase of 2.93%. While their overall decline is less severe, uncertainties surrounding their true wild distribution and local extinction risks justify their classification as NT.
Discussion
Factors determining the distribution of Solanum wild relatives
The distribution and suitable habitat of the species studied are largely influenced by key abiotic factors such as rainfall, temperature, and soil properties. Climate variables (e.g. temperature and precipitation) and edaphic conditions play a fundamental role in determining species presence in a given environment (Lewis et al, 2017). However, the ability of these species to persist in complex environment also depends on their interactions with biotic factors – competition, parasitism, commensalism – and on dispersal constraints that were not included in our models. Moreover, the models did not consider species’ phenotypic plasticity and evolutionary adaptation to changing environments (Pidwirny, 2006). Integrating these aspects in future modelling efforts could substantially improve the predictive accuracy and ecological realism of species distribution models, especially under changing climate scenarios.
In this study, we found that the distribution patterns of the ten Solanum species were primarily influenced by bioclimatic variables, with isothermality emerging as the most dominant factor. Edaphic variables also played a role, albeit with a relatively smaller contribution to the distribution of each species.
Our findings align with those of Manda et al (2022) who identified isothermality as the second most important factor when modelling the potential impact of climate change on Vigna wild relatives. Similar patterns in the influence of bioclimatic and soil variables have been reported for tree species such as Balanites aegyptiaca (L.) Delile (Chérif et al, 2022) in Chad, Fontainea species (Brunton et al, 2023) in Australia and palm species in Benin (Idohou et al, 2017; Salako et al, 2019). In the case of wild relatives of cultivated Solanum leafy vegetables, their responses to soil characteristics varied among species. Indeed, several studies have highlighted the high nutritional value of African indigenous vegetables, including Solanum species, attributing their richness in nutrients to underlying edaphic conditions (Chinedu et al, 2011; Keatinge et al, 2011) . This relationship suggests that soil properties play a significant role in shaping the nutrient profile of these plants. Additionally, the observed variability among species likely reflects inherent physiological traits that influence water requirements and nutrient uptake. Since Solanum seed germination is sensitive to environmental stress, favourable soil and moisture conditions are essential for successful germination and, consequently, for the long-term persistence of the species in a given habitat (Stanton et al, 2012). These factors should be carefully considered when it comes to cultivation or conservation. Understanding their responses to environmental changes is therefore essential for designing effective conservation plans. As reported by Aksoy et al (2021), evaluating the impact of climate change is crucial for the conservation of S. tuberosum. Similarly, Ogundola et al (2023) reported that soil characteristics, particularly silty loam soils at a depth of 2cm, enhance the germination and viability of S. nigrum seeds. In our study, the most influential soil factors included exchangeable acidity, exchangeable magnesium, bulk density, silt content, and soil pH. However, further research is necessary to better understand how these specific edaphic parameters affect seed germination, viability and overall fitness of Solanum species under both current and future environmental conditions.
Suitable areas of Solanum wild relatives and climate change
The suitable habitats of wild relatives of cultivated Solanum leafy vegetables are likely to be largely unstable in the coming decades, reinforcing our first hypothesis that abiotic factors are the main drivers of the current and future distribution of wild Solanum species.
Climate change is predicted to increase the temperature by 1.5–2°C by 2100 in West Africa, and to cause an uneven distribution of precipitation (IPCC, 2021). Based on these projections, the suitable habitats of the ten wild Solanum species are likely to be severely altered by the 2055 and 2085time horizons under both SSP3-7.0 and SSP5-8.5 scenarios. As noted by Guisan and Thuiller (2005), several factors (population dynamics, migration, local adaptations, ecological interactions, disease prevalence and human intervention) can influence the distribution of a species. Our results indicate substantial habitat losses for nearly all species by both the 2055 and 2085 horizons under the two scenarios. Surprisingly, a rise in the number of suitable areas was observed for wild S. melongena and S. dasyphyllum. This suggests that climate change could have a positive impact on the potential distribution of these species. This expansion could imply an increase in the potential cultivation area for domesticated or improved forms of these species. However, further agronomic and socioeconomic studies would be necessary to confirm this. Under the SSP5-8.5, the situation appears particularly critical across both time horizons, with predicted high habitat losses. These findings align with numerous other studies which reported shifts in the distribution of many CWR. For instance, Manda et al (2022) reported major changes in the distribution of Vigna wild relatives in Benin. Similarly, findings in Southern Africa indicated that the majority of regionally priority CWR are expected to be negatively impacted by climate change, threatening their survival (Magos Brehm et al, 2022). Additionally, van Treuren et al (2020) demonstrated a reduction of suitable areas for CWR in the Netherlands. Seed dispersal is a crucial ecological mechanism that could either hinder or facilitate plant species’ response to climate change (Öckinger et al, 2010). According to the same author, species with limited dispersal ability would be the most affected by the environmental changes compared to those with strong dispersal capacity. Solanum species are dispersed by mammals, bats, birds, human-induced actions, as well as wind and water flow (Roberts and Florentine, 2022), which are predicted to be highly disturbed by climate change, potentially disrupting natural dispersal processes. Besides, in the Sudano-Guinean zone, habitat fragmentation driven by agricultural expansion and rapid population growth has already impeded natural dispersal pathways (Neuenschwander & Adomou, 2017; Abdul Aziz et al, 2024). Consequently, natural dispersion may not be efficient with humans as a major dispersing agent. Compounding this issue, invasive species pose a significant threat to native biodiversity, challenging both ecological resilience and conservation efforts. In Benin, many studies showed the detrimental impacts of invasive species on native species particularly in PA (Gbètoho et al, 2017) and, at the same time, models predicted their capacity to thrive in the changing climate (Fandohan et al, 2015). This is an important aspect to consider since most wild Solanum species are herbaceous and, thus, highly susceptible to displacement by aggressive invaders such as Chromolaena odorata (L.) R.M.King & H.Rob.
Solanum wild relatives and their conservation within protected areas
The results showed that, with a few exceptions in the Sudano-Guinean and Guineo-Congolian zones, most existing PA offer limited potential for conserving the target Solanum species. Future projections confirmed these patterns, although localised expansion or contraction may occur. The destruction of natural habitats on a large scale, combined with climate change, is one of the main threats to plant species (Hudson et al, 2014), and this includes CWR (Hunter et al, 2012; Magos Brehm et al, 2022; Maxted et al, 2012). Protected areas are often cited as refuges for threatened species (Le Saout et al, 2013); their ability to maintain populations within defined boundaries offers a valuable conservation mechanism, especially in regions undergoing rapid environmental change (Le Saout et al, 2013; Mao et al, 2020). However, the mere presence of a species within a PA does not guarantee its protection. More often than not, this represents passive in situ conservation, whereby species may persist without any specific monitoring or management actions. In the context of accelerating climate change, there is a critical need for active in situ conservation, which involves the direct management, monitoring and support of populations over time, to ensure long-term effectiveness. Our findings align with those of Manda et al (2022), who reported that the existing PA network was inefficient in conserving wild Vigna species, a trend also observed in several other countries (e.g. Davis et al, 2019; Ratnayake et al, 2021). Conversely, Idohou et al (2017) highlighted the potential of PA for conserving palm species in Benin. However, whether such potential persists under future climate scenarios remains uncertain and warrants further investigation. Despite being considered refuges for many plant species, our projections suggest that both the current and future distributions of the studied Solanum species may fall outside the existing PA network. This supports the hypothesis that current PA may not be sufficient to conserve wild Solanum habitats under climate change. Therefore, it is urgent to identify and prioritize additional conservation areas that are more likely to remain suitable in the future. Sub-Saharan Africa, particularly its arid and semi-arid zones, has been identified as one of the most climate-vulnerable regions in the world (Sintayehu, 2018), with native species facing multiple threats. Under such pressures, the distribution of most wild Solanum species is expected to become increasingly unstable in future climate scenarios. As a result, proactive conservation actions are essential (Schuster et al, 2023). We thus emphasize the need to conserve wild Solanum not only within PA networks but also beyond them, through mechanisms such as the IUCN’s Other Effective Area-Based Conservation Measures (OECMs). In particular, agroforestry systems and home gardens represent promising complementary conservation environments. If properly designed and supported, these managed landscapes could host viable populations of wild relatives and provide additional resilience to climate stressors.
Implications for better management of Solanum wild relatives
In this study, we investigated the threats to the conservation of ten wild Solanum species under different future climate scenarios. Many studies demonstrated the great importance of CWR worldwide (e.g. Ng'uni et al, 2019; Tas et al, 2019; Maxted and Magos Brehm, 2023). Because of the key role they play in crop improvement and food production, there is a global call for the conservation and preservation of these resources (Maxted et al, 2010; Maxted et al, 2012). Assessment of the current and future distribution of Solanum species under climate change revealed a decline in suitable habitats for the majority of the species. Furthermore, the assessment based on IUCN Red List Categories and Criteria indicated that most species in Benin are threatened, underscoring their high risk of genetic erosion, a pattern similarly observed among numerous CWR taxa worldwide. Our study identified priority areas for target Solanum species conservation as suggested by Maxted et al (2009). The target species to be prioritized for conservation include S. anguivi, S. erianthum, S. anomalum and S. terminale (VU, EN and CR) since they are likely to continue losing suitable areas of distribution. Monitoring population status and habitat conditions, restoring degraded ecosystems, and analyzing genetic diversity are all essential steps for the effective conservation of Solanum species. Monitoring enables the early detection of population declines, while restoration efforts help ensure that species can persist in their natural habitats. Preserving a broad spectrum of genetic diversity is crucial for the long-term adaptability and survival of species. It also ensures that these genetic resources remain available to plant breeders, helping to improve crops and ensure food security. Ecogeographic diversity, which integrates environmental and geographical variation, can serve as a useful proxy for capturing genetic variation (Parra-Quijano et al, 2012). These species could also be protected in several sacred forests throughout the country. Although they are limited in size, sacred forests are often better conserved than most classified forests (Adomou et al, 2007), making them suitable refuges for the conservation of Solanum taxa through introduction or reintroduction programmes. To minimize ecological risks, such introductions should occur within each species' historical distribution range. In parallel, ex situ conservation is a valuable complementary strategy, particularly for species with critically reduced habitats. However, the long-term success of these measures depends on a better understanding of the ecological requirements of each species, including their capacity to adapt and their productivity under changing environmental conditions.
Conservation in the Wari-Maro and Belefoungou forests could be more effective than in the semi-deciduous forests of southern Benin, such as the Lama reserve or sacred forests, primarily because many of the Solanum species studied are heliophilous, meaning they thrive in environments with high light availability. Unlike in dense, closed-canopy forests, where light penetration is limited, the drier, more open structure of the Wari-Maro and Belefoungou forests provides favourable microhabitats for these species that demand light. These conditions support germination, growth and reproductive success, thereby enhancing their long-term survival prospects in these forest systems. Furthermore, classified forest reserves, including sacred forests, have decreased in recent decades, implying the loss and fragmentation of suitable habitats for many taxa in Benin (Alohou et al, 2017). According to Pinto et al (2024), the continued loss of suitable areas may result in significant genomic erosion for metapopulation taxa. The impact of these losses on Solanum species should also be investigated. Finally, the occurrence of invasive species in natural habitats needs to be considered when defining these strategies.
Limitations of the approach
In this study, we investigated the current distribution of ten wild Solanum species in Benin and made predictions for their future distribution. The model accuracy and robustness were assessed by computing the AUC and TSS values of the final MaxEnt outputs (Allouche et al, 2006). However, we did not include seed dispersal limitations or constraints that could hinder the spread of species across landscapes. Furthermore, we did not account for physiological, phenological and morphological traits in our models (Koebsch et al, 2019; Agounde et al, 2025).
Although the SDM-based projections offer valuable insights into the potential impact of climate change on the distribution of wild Solanum species in Benin, it is important to acknowledge that our assessment is still preliminary. The absence of data on demographic trends and population viability may result in certain taxa being underestimated as vulnerable. Consequently, these results should be interpreted as indicative rather than definitive. Future assessments will require the integration of ecological and population-level data in order to better reflect extinction risk and guide conservation priorities.
Conclusion
Developing an effective conservation strategy for a given species requires accurate information on the species' distribution range as a fundamental basis. Using SDM tools and IUCN criteria, we assessed the potential distribution, habitat suitability and preliminary conservation status of ten wild Solanum species under current and projected climatic conditions. Overall, most of the species currently have a wide distribution in the Sudano-Guinean and Guineo-Congolian zones of Benin. Significant declines in potentially suitable habitats were found in the two scenarios for 2055 and 2085. However, for more accurate projections, we recommend additional distribution studies taking into account physiological, phenological and morphological data.
The study demonstrated that the current protected area network is ineffective in ensuring the long-term survival of these species in the face of changing climatic conditions. Therefore, conservation efforts should prioritize active in situ strategies, such as restoring degraded habitats, reinforcing natural populations within their historical ranges and integrating climate-smart management plans. Given the limited coverage and effectiveness of existing mechanisms in Benin, such actions are urgently needed. In parallel, ex situ conservation measures, including seedbanks and living collections, should complement in situ efforts to preserve the genetic diversity of these species and facilitate their potential use in future food security initiatives.
Acknowledgements
This research work was supported by European Commission Directorate-General for International Partnerships through the Regional Academic Exchange for Enhanced Skills in Fragile Ecosystems Management in Africa (Grant Number Nr 2017-2861/001-001) and the PhD fellowship from the Organization for Women in Science for the Developing World (Grant Number: 3240314469) provided to Ahuéfa Mauricel Kégbé. Ahuéfa Mauricel Kégbé is very grateful to Dr Valère Salako and Dr Ablaye Ngom for their encouragement and support during the implementation of this research. Rodrigue Idohou acknowledges support for BITC research and training visits from the John Roger Seamans Biodiversity Foundation, which enabled collaboration on this paper.
Author contributions
Ahuéfa Mauricel Kégbé, Rodrigue Idohou: conceptualization, methodology, visualization, software, resources, formal analysis, writing original draft, writing, review and editing; Birane Dieng: writing original first draft; Gafarou Agounde: software, formal analysis, resources, writing original first draft; Anthony Egeru: review and editing; Kandioura Noba: review and editing; Achille Ephrem Assogbadjo: supervision, software, writing, review and editing. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Supplemental Table 1. The ecology, potential use and conservation status of the Solanum species
Supplemental Table 2: Species occurrences used in ecology niche modelling
Supplemental Table 3. Description of the environmental variables
Supplemental Table 4. Correlation analysis
Supplemental Table 5. Dynamic of the distribution of Solanum species in the whole study area
Supplemental Table 6. Dynamic of the distribution of Solanum species in protected areas
Supplemental Table 7. Spatial reduction of extent of richness class in the whole study area
Supplemental Table 8. Spatial reduction of extent of richness class in the protected areas
Supplemental Table 9. Threat and distribution range of the ten Solanum genus in Benin
Supplemental Table 10. Conservation status of Solanum in Benin
References
Abdul Aziz S., Émeline Sêssi Pelagie A., Séverin B., Ogoulonou Rodrigue B., Bertrand A., Samadori Sorotori Honoré B. (2024). Land use/land cover and plant community dynamics in the Benin's forest reserves: The effectiveness of participatory forest management Trees, Forests and People 16: 100543. https://doi.org/10.1016/j.tfp.2024.10054
Adomou, A. (2005). Vegetation patterns and environmental gradients in Benin: implications for biogeography and conservation. Ph.D. Thesis, Wageningen University, The Netherlands.
Adomou, A. C., Yedomonhan, H., Sinsin, B., and Van der Maesen, L. J. G. (2007). Distribution des aires protégées et conservation de la flore en république du Bénin: Notulae Florae Beninensis 11.
Agounde, G., Salako, K.V., Idohou, R.A., Sode, A.I., Mensah, S., Dimobe, K., Assogbadjo, A.E., Glèlè Kakaï, R. (2025). Climate change may shift diet of the African savanna elephant: Preliminary results for 14 food tree and shrub species in the WAPOK transboundary ecosystem, West-Africa. Global Ecology and Conservation, 58, p.e03468. https://doi.org/10.1016/j.gecco.2025.e03468
Ahmad, S., Yang, L., Khan, T. U., Wanghe, K., Li, M. and Luan, X. (2020). Using an ensemble modelling approach to predict the potential distribution of Himalayan gray goral (Naemorhedus goral bedfordi) in Pakistan. Glob. Ecol. Conserv., 21, e00845. doi: https://doi.org/10.1016/j.gecco.2019.e00845
Akoègninou, A., van der Burg, W. and van der Maesen, L. (2006). Flore Analytique du Bénin: Wageningen: Backhuys Publishers
Aksoy, E., Demirel, U., Bakhsh, A., Zia, M. A. B., Naeem, M., Saeed, F., ... and Çalışkan, M. E. (2021). Recent advances in potato (Solanum tuberosum L.) breeding. Advances in Plant Breeding Strategies: Vegetable Crops: Volume 8: Bulbs, Roots and Tubers, 409-487. https://doi.org/10.1007/978-3-030-66965-2_10
Allouche, O., Tsoar, A. and Kadmon, R. (2006). Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol., 43(6), 1223-1232. https://doi.org/10.1111/j.1365-2664.2006.01214.x
Alohou, E. C., Gbemavo, D. S. J. C., Mensah, S., and Ouinsavi, C. (2017). Fragmentation of forest ecosystems and connectivity between Sacred Groves and Forest Reserves in southeastern Benin, West Africa. Tropical Conservation Science, 10, 1940082917731730.
Barbet-Massin, M., Jiguet, F., Albert, C.H. and Thuiller, W. (2012), Selecting pseudo-absences for species distribution models: how, where and how many?. Methods in Ecology and Evolution, 3: 327-338. https://doi.org/10.1111/j.2041-210X.2011.00172.x
Boucher, O., Servonnat, J., Albright, A. L., Aumont, O., Balkanski, Y., Bastrikov, V., Bekki, S., Bonnet, R., Bony, S. and Bopp, L. (2020). Presentation and evaluation of the IPSL-CM6A-LR climate model. J. Adv. Model. Earth Syst., 12(7), e2019MS002010. doi: https://doi.org/10.1029/2019MS002010
Brehm, J., Gaisberger, H., Kell, S., Parra-Quijano, M., Thormann, I., Dulloo, M. E., and Maxted, N. (2022). Planning complementary conservation of crop wild relative diversity in southern Africa. Diversity and Distributions, 28, 1358–1372. https://doi.org/10.1111/ddi.13512
Breiman, L. (2001). Random forests. Machine learning, 45, 5-32. doi:https://doi.org/10.1023/A:1010933404324
Brooks, T. M., Pimm, S. L., Akçakaya, H. R., Buchanan, G. M., Butchart, S. H., Foden, W., Hilton-Taylor, C., Hoffmann, M., Jenkins, C. N. and Joppa, L. (2019). Measuring terrestrial area of habitat (AOH) and its utility for the IUCN Red List. Trends Ecol. Evol., 34(11), 977-986. doi: https://doi.org/10.1016/j.tree.2019.06.009
Brown, J. L. (2014). SDM toolbox: a python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol., 5(7), 694-700. https://doi.org/10.1111/2041-210X.12200
Brunton, A. J., Conroy, G. C., Schoeman, D. S., Rossetto, M. and Ogbourne, S. M. (2023). Seeing the forest through the trees: Applications of species distribution models across an Australian biodiversity hotspot for threatened rainforest species of Fontainea. Glob. Ecol. Conserv., 42, e02376. doi:https://doi.org/10.1016/j.gecco.2023.e02376
Burgess, N., Küper, W., Mutke, J., Brown, J., Westaway, S., Turpie, S., Meshack, C., Taplin, J., McClean, C. and Lovett, J. C. (2005). Major gaps in the distribution of protected areas for threatened and narrow range Afrotropical plants. Biodivers. Conserv., 14, 1877-1894. doi:https://doi.org/10.1007/S40531-004-1299-2
Bussmann, R.W., Paniagua-Zambrana, N.Y., and Njoroge, G.N. (2021). Solanum aculeastrum Dunal Solanum anguivi Lam. Solanum incanum L. Solanum nigrum L. Solanaceae. In: Bussmann, R.W. (eds) Ethnobotany of the Mountain Regions of Africa. Ethnobotany of Mountain Regions. Springer, Cham. https://doi.org/10.1007/978-3-030-38386-2
Castañeda-Álvarez, N. P., De Haan, S., Juárez, H., Khoury, C. K., Achicanoy, H. A., Sosa, C. C., ... and Spooner, D. M. (2015). Ex situ conservation priorities for the wild relatives of potato (Solanum L. section Petota). PLoS One, 10(4), e0122599. https://doi.org/10.1371/journal.pone.0122599
Chérif, A., Sodé, A., Houndonougbo, J., Idohou, R., Fandohan, A., Kakaï, R. G. and Assogbadjo, A. (2022). Habitat suitability modeling for the conservation and cultivation of the multipurpose fruit tree, Balanites aegyptiaca L., in the Republic of Chad, Sahel. Model. Earth Syst. Environ., 8(4), 4953-4963. doi:https://doi.org/10.1007/s40808-022-01416-4
Chinedu, S. N., Olasumbo, A. C., Eboji, O. K., Emiloju, O. C., Arinola, O. K. and Dania, D. I. (2011). Proximate and phytochemical analyses of Solanum aethiopicum L. and Solanum macrocarpon L. fruits. Research Journal of Chemical Sciences, 1(3), 63-71.
Coulibaly M., Idohou R., Akohoue F., Peterson A.T., Sawadogo M., and Achigan-Dako E.G. (2021). (2022). Coupling genetic structure analysis and ecological-niche modeling in Kersting’s groundnut in West Africa. Scientific reports, 12(1), 5590.
Daï, E. H., Houndonougbo, J. S. H., Idohou, R., Ouédraogo, A., Kakaï, R. G., Hotes, S. and Assogbadjo, A. E. (2023). Modeling current and future distribution patterns of Uvaria chamae in Benin (West Africa): Challenges and opportunities for its sustainable management. Heliyon, 9(2). doi:https://doi.org/10.1016/j.heliyon.2023.e13658
Dassou G.H, Agoundé G, Akouété P, Favi GA, Kpétikou GC, Salako K.V, Ouachinou J, Makponsè J, Kouyaté A.M, Sari I, Glèlè Kakaï R.L, Yédomonhan H, and Adomou A.C (2024) Past, present, and future potential distributions of the African multipurpose tree Detarium senegalense (Fabaceae). Plant Ecology and Evolution 157(3): 343-357. https://doi.org/10.5091/plecevo.122470
Davis, A. P., Chadburn, H., Moat, J., O’Sullivan, R., Hargreaves, S. and Nic Lughadha, E. (2019). High extinction risk for wild coffee species and implications for coffee sector sustainability. Sci. Adv., 5(1), eaav3473. doi:https://doi.org/10.1126/sciadv.aav3473
Fandohan, A. B., Oduor, A. M. O., Sodé, A. I., Wu, L., Cuni-Sanchez, A., Assédé, E. and Gouwakinnou, G. N. (2015). Modeling vulnerability of protected areas to invasion by Chromolaena odorata under current and future climates. Ecosyst. Health Sustain., 1(6), 1-12. doi:https://doi.org/10.1890/EHS45-0003.1
Feng, X., Liang, Y., Gallardo, B. and Papeş, M. (2020). Physiology in ecological niche modeling: using zebra mussel's upper thermal tolerance to refine model predictions through Bayesian analysis. Ecography, 43(2), 270-282. doi: https://doi.org/10.1111/ecog.04627
Friedman, J., Hastie, T. and Tibshirani, R. (2000). ADDITIVE LOGISTIC REGRESSION: A STATISTICAL VIEW OF BOOSTING. Ann. Stat., 28(2), 337-407. doi:https://doi.org/10.1214/aos/1016218223
Gbètoho, A. J., Aoudji, A. K., Roxburgh, L. and Ganglo, J. C. (2017 ). Assessing the suitability of pioneer species for secondary forest restoration in Benin in the context of global climate change. Bois for. trop., 332, 43-55. doi:https://doi.org/10.19182/bft2017.332.a31332
Gebhardt, C. (2016). The historical role of species from the Solanaceae plant family in genetic research. Theor. Appl. Genet., 129, 2281-2294. doi:https://doi.org/10.1007/s00122-016-2804-1
Guisan, A., and Thuiller, W. (2005). Predicting species distribution: offering more than simple habitat models. Ecology letters, 8(9), 993-1009.
Hijmans, R. J. and Elith, J. (2017). Species distribution modeling with R. R Cran Project.
Hounsou-Dindin, G., Idohou, R., Agre, P., Hounkpèvi, A., Adomou, A. C., Assogbadjo, A. E. and Kakaï, R. G. (2023). Habitat range shift and prediction of the potential future distribution of Ricinodendron heudelotii (Baill.) Heckel in Benin (West Africa). Heliyon, 9(9). doi:https://doi.org/10.1016/j.heliyon.2023.e20199
Hudson, L. N., Newbold, T., Contu, S., Hill, S. L., Lysenko, I., De Palma, A., Phillips, H. R., Senior, R. A., Bennett, D. J. and Booth, H. (2014). The PREDICTS database: a global database of how local terrestrial biodiversity responds to human impacts. Ecol. Evol., 4(24), 4701-4735. doi: https://doi.org/10.1002/ece3.1303
Hunter, D., Maxted, N., Heywood, V., Kell, S. and Borelli, T. (2012). Protected areas and the challenge of conserving crop wild relatives. Parks, 18(1), 87.
Idohou, R., Odoulami, R., Houehanou, T., & Assogbadjo, A. (2025). Top priority crop wild relatives exhibit different resilience responses to climate change in Benin (West Africa). Journal for Nature Conservation, 83, 126769.
Idohou, R., Assogbadjo, A. E., Fandohan, B., Gouwakinnou, G. N., Glele Kakai, R. L., Sinsin, B., & Maxted, N. (2013). National inventory and prioritization of crop wild relatives: case study for Benin. Genetic Resources and Crop Evolution, 60, 1337-1352.
Idohou, R., Assogbadjo, A. E., Kakai, R. G. and Peterson, A. T. (2017). Spatio-temporal dynamic of suitable areas for species conservation in West Africa: eight economically important wild palms under present and future climates. Agrofor. Syst., 91, 527-540. doi:https://doi.org/10.1007/S40457-016-9955-6
IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2391 pp. doi:https://doi.org/10.1017/9781009157896.
IUCN (2024) The IUCN Red List of Threatened Species. Version 2024-2. https://www.iucnredlist.org ISSN 2307-8235
Jarvis, A., Lane, A. and Hijmans, R. J. (2008). The effect of climate change on crop wild relatives. Agric. Ecosyst. Environ., 126(1-2), 13-23. doi:https://doi.org/10.1016/j.agee.2008.01.013
Karger, D. N., Conrad, O., Böhner, J., Kawohl, T., Kreft, H., Soria-Auza, R. W., Zimmermann, N. E., Linder, H. P. and Kessler, M. (2017). Climatologies at high resolution for the earth’s land surface areas. Sci. Data, 4(1), 1-20. doi:https://doi.org/10.1038/sdata.2017.122
Keatinge, J., Yang, R.-Y., Hughes, J. d. A., Easdown, W. and Holmer, R. (2011). The importance of vegetables in ensuring both food and nutritional security in attainment of the Millennium Development Goals. Food Secur., 3(4), 491-501. doi:https://doi.org/10.1007/S42571-011-0150-3
Koebsch, F., Winkel, M., Liebner, S., Liu, B., Westphal, J., Schmiedinger, I., Spitzy, A., Gehre, M., Jurasinski, G. and Köhler, S. (2019). Sulfate deprivation triggers high methane production in a disturbed and rewetted coastal peatland. Biogeosciences, 16(9), 1937-1953. doi:https://doi.org/10.5194/bg-16-1937-2019
Lala, S., Amri, A. and Maxted, N. (2018). Towards the conservation of crop wild relative diversity in North Africa: checklist, prioritisation and inventory. Genet. Resour. Crop Evol., 65(1), 113-124. doi:https://doi.org/10.1007/S40722-017-0513-5
Le Saout, S., Hoffmann, M., Shi, Y., Hughes, A., Bernard, C., Brooks, T. M., Bertzky, B., Butchart, S. H., Stuart, S. N. and Badman, T. (2013). Protected areas and effective biodiversity conservation. Science, 342(6160), 803-805. doi:https://doi.org/10.1126/science.1239268
Lewis, J. S., Farnsworth, M. L., Burdett, C. L., Theobald, D. M., Gray, M. and Miller, R. S. (2017). Biotic and abiotic factors predicting the global distribution and population density of an invasive large mammal. Sci. Rep., 7(1), 44152. doi:https://doi.org/10.1038/srep44152
MAEP. (2020). Indicateurs macroeconomiques sur le secteur agricole au Benin: Direction de la Statisque Agricole, Republique du Bénin.
Magioli, C., and Mansur, E. (2005). Eggplant (Solanum melongena L.): tissue culture, genetic transformation and use as an alternative model plant. Acta Botanica Brasilica, 19, 139-148. https://doi.org/10.1590/S0102-33062005000100013
Manda, L., Idohou, R., Assogbadjo, A. E. and Agbangla, C. (2022). Climate change reveals contractions and expansions in the distribution of suitable habitats for the neglected crop wild relatives of the Genus Vigna (Savi) in Benin. Front. conserv. sci., 3, 870041. doi:https://doi.org/10.3389/fcosc.2022.870041
Mao, L., Li, M. and Shen, W. (2020). Remote sensing applications for monitoring terrestrial protected areas: Progress in the last decade. Sustainability, 12(12), 5016. doi:https://doi.org/10.3390/su12125016
Masson-Delmotte, V., Zhai, P., Pörtner, H., Roberts, D., Skea, J. and Shukla, P. R. (2022). Global Warming of 1.5 C: IPCC special report on impacts of global warming of 1.5 C above pre-industrial levels in context of strengthening response to climate change, sustainable development, and efforts to eradicate poverty (https://doi.org/10.1017/9781009157940): Cambridge University Press.
Maxted, N., Ford-Lloyd, B. V., Jury, S., Kell, S. and Scholten, M. (2006). Towards a definition of a crop wild relative. Biodivers. Conserv., 15(8), 2673-2685. doi:https://doi.org/10.1007/S40531-005-5409-6
Maxted, N., Kell, S. and Brehm, J. M. (2009). Commission on genetic resources for food and agriculture. Establishment of a global network for the in-situ conservation of crop wild relatives: status and needs. Background study paper(39), 212.
Maxted, N., Kell, S., Ford-Lloyd, B., Dulloo, E. and Toledo, Á. (2012). Toward the systematic conservation of global crop wild relative diversity. Crop Sci., 52(2), 774-785. doi:https://doi.org/10.2135/cropsci2011.08.0415
Maxted, N., Kell, S., Toledo, Á., Dulloo, E., Heywood, V., Hodgkin, T., Hunter, D., Guarino, L., Jarvis, A. and Ford-Lloyd, B. (2010). A global approach to crop wild relative conservation: securing the gene pool for food and agriculture. Kew Bulletin, 65(4), 561-576. doi:https://doi.org/10.1007/S42225-011-9253-4
Maxted, N. and Magos Brehm, J. (2023). Maximizing the crop wild relative resources available to plant breeders for crop improvement. Front. sustain. food syst., 7, 1010204. doi:https://doi.org/10.3389/fsufs.2023.1010204
Meyer, D. and Wien, F. (2001). Support vector machines. R News, 1(3), 23-26.
Montoya-Jiménez JC, Valdez-Lazalde JR, Ángeles-Perez G, De Los Santos-Posadas HM, and Cruz-Cárdenas G (2022). Predictive capacity of nine algorithms and an ensemble model to determine the geographic distribution of tree species. iForest-Biogeosciences and Forestry 15(5): 363. https://doi.org/10.3832/ifor4084-015
Naimi, B. (2017). Package ‘usdm’. Uncertainty analysis for species distribution models. Wien: www.cran.r-project.org.
Neuenschwander P., Adomou A.C. (2017). Reconstituting a rainforest patch in southern Benin for the protection of threatened plants Nature Conservation 21: 57-82.https://doi.org/10.3897/natureconservation.21.13906
Ng'uni, D., Munkombwe, G., Mwila, G., Gaisberger, H., Brehm, J. M., Maxted, N., Kell, S. and Thormann, I. (2019). Spatial analyses of occurrence data of crop wild relatives (CWR) taxa as tools for selection of sites for conservation of priority CWR in Zambia. Plant Genetic Resources, 1-12. doi:https://doi.org/10.1017/S4479262118000497
Öckinger, E., Schweiger, O., Crist, T. O., Debinski, D. M., Krauss, J., Kuussaari, M., Petersen, J. D., Pöyry, J., Settele, J. and Summerville, K. S. (2010). Life-history traits predict species responses to habitat area and isolation: a cross-continental synthesis. Ecol. Lett., 13(8), 969-979. doi:https://doi.org/10.1111/j.1461-0248.2010.01487.x
Ogundola, A. F., Afolayan, A. J., and Bvenura, C. (2023). Solanum nigrum Seed viability and germination, and soil modulation effect on seedling emergence. In Sustainable Uses and Prospects of Medicinal Plants (pp. 133-143). CRC Press.
Okokon, J. E., Davies, K. O., Amazu, L. U. and Umoh, E. E. (2017). Anti-inflammatory activity of leaf extract of Solanum anomalum. Journal of Medicinal Herbs, 7(4), 243-249.
Oyinloye, O.E., Ajayi, A.M. and Ademowo O.G. (2022). Solanum dasyphyllum leaf extract reduces inflammation in carrageenan-induced air pouch in rats by inhibition of cyclooxygenase-2 and inducible nitric oxide synthase. Nutrire 47, 24 https://doi.org/10.1186/s41110-022-00175-7
Parra-Quijano, M., Iriondo, J.M. & Torres, E. Ecogeographical land characterization maps as a tool for assessing plant adaptation and their implications in agrobiodiversity studies. Genetic Resources Crop Evolution 59, 205–217 (2012). https://doi.org/10.1007/s10722-011-9676-7
Parviainen, M., Luoto, M., Ryttäri, T. and Heikkinen, R. K. (2008). Modelling the occurrence of threatened plant species in taiga landscapes: methodological and ecological perspectives. J. Biogeogr., 35(10), 1888-1905. doi:https://doi.org/10.1111/j.1365-2699.2008.01922.x
Phillips, S. J., Anderson, R. P., Dudik, M., Schapire, R. E. and Blair, M. E. (2017). Opening the black box: An open-source release of Maxent. Ecography, 40(7), 887-893. doi:https://doi.org/10.1111/ecog.03049
Phillips, S. J., Anderson, R. P. and Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecol. Modell., 190(3-4), 231-259. doi:https://doi.org/10.1016/j.ecolmodel.2005.03.026
Pidwirny, M. (2006). Abiotic factors and the distribution of species. Fundamentals of Physical Geography.
Pilling, D., Bélanger, J., Diulgheroff, S., Koskela, J., Leroy, G., Mair, G., and Hoffmann, I. (2020). Global status of genetic resources for food and agriculture: challenges and research needs. Genetic Resources 1 (1), 4-16. doi: https://doi.org/10.46265/genresj.2020.1.4-16.
Pinto, A. V., Hansson, B., Patramanis, I., Morales, H. E., and van Oosterhout, C. (2024). The impact of habitat loss and population fragmentation on genomic erosion. Conservation Genetics, 25(1), 49-57. https://doi.org/10.1007/s10592-023-01548-9
Ramírez-Villegas, J., Khoury, C., Jarvis, A., Debouck, D. G., and Guarino, L. (2010). A gap analysis methodology for collecting crop genepools: a case study with Phaseolus beans. PloS one, 5(10), e13497. https://doi.org/10.1371/journal.pone.0013497
Ratnayake, S. S., Kariyawasam, C. S., Kumar, L., Hunter, D. and Liyanage, A. (2021). Potential distribution of crop wild relatives under climate change in Sri Lanka: implications for conservation of agricultural biodiversity. Current Research in Environmental Sustainability, 3, 100092. doi:https://doi.org/10.1016/j.crsust.2021.100092
Roberts, J. and Florentine, S. (2022). Biology, distribution and management of the globally invasive weed Solanum elaeagnifolium Cav (silverleaf nightshade): A global review of current and future management challenges. Weed Res., 62(6), 393-403. doi:https://doi.org/10.1111/wre.12556
R Team (2023). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing (Version 4.0.3.). Vienna, Austria. Retrieved from https://www.R-project.org/
Salako, V. K., Vihotogbé, R., Houéhanou, T., Sodé, I. A. and Glèlè Kakaï, R. (2019). Predicting the potential impact of climate change on the declining agroforestry species Borassus aethiopum Mart. in Benin: a mixture of geostatistical and SDM approach. Agrofor. Syst., 93, 1513-1530. doi:https://doi.org/10.1007/S40457-018-0262-2
Samuels, J. (2015). Biodiversity of food species of the Solanaceae family: a preliminary taxonomic inventory of subfamily Solanoideae. Resources, 4(2), 277-322. doi:https://doi.org/10.3390/resources4020277
Sarma, H. and Sarma, A. (2011). Solanum nigrum L., a nutraceutical enriched herb or invasive weed? Paper presented at the International Conference on Environment and BioScience IPCBEE.
Schuster, R., Buxton, R., Hanson, J. O., Binley, A. D., Pittman, J., Tulloch, V., La Sorte, F. A., Roehrdanz, P. R., Verburg, P. H. and Rodewald, A. D. (2023). Protected area planning to conserve biodiversity in an uncertain future. Conserv. Biol., 37(3), e14048. doi:https://doi.org/10.1111/cobi.14048
Sintayehu, D. W. (2018). Impact of climate change on biodiversity and associated key ecosystem services in Africa: a systematic review. Ecosyst. Health Sustain., 4(9), 225-239. doi:https://doi.org/10.1080/20964129.2018.1530054
Stanton, R., Wu, H. and Lemerle, D. (2012). Factors affecting silverleaf nightshade (Solanum elaeagnifolium) germination. Weed Sci., 60(1), 42-47. doi:https://doi.org/10.1614/WS-D-11-00105.1
Steffen, W., Broadgate, W., Deutsch, L., Gaffney, O. and Ludwig, C. (2015). The trajectory of the Anthropocene: the great acceleration. Anthr. Rev., 2(1), 81-98. doi:https://doi.org/10.1177/2053019614564785
Stolton, S., Maxted, N., Ford-Lloyd, B., Kell, S. and Dudley, N. (2006). Food Stores: Using Protected Areas to Secure Crop Genetic Diversity; Worldwide Fund for Nature: Woking, UK.
Syfert, M. M., Castañeda-Álvarez, N. P., Khoury, C. K., Särkinen, T., Sosa, C. C., Achicanoy, H. A., Bernau, V., Prohens, J., Daunay, M. C. and Knapp, S. (2016). Crop wild relatives of the brinjal eggplant (Solanum melongena): Poorly represented in genebanks and many species at risk of extinction. Am. J. Bot., 103(4), 635-651. doi:https://doi.org/10.3732/ajb.1500539
Tas, N., West, G., Kircalioglu, G., Topaloglu, S. B., Phillips, J., Kell, S. and Maxted, N. (2019). Conservation gap analysis of crop wild relatives in Turkey. Plant Genetic Resources, 1-10. doi:https://doi.org/10.1017/S4479262118000564
Toffa, Y., Idohou, R. and Fandohan, A. B. (2022). Modélisation de la distribution des espèces en Afrique: état de l'art et perspectives. Physio-Géo. Géographie physique et environnement, 17, 43-65. doi:https://doi.org/10.4000/physio-geo.13738
UNEP-WCMC (2023) Protected areas map of the world, https://www.protectedplanet.net/en
van Treuren, R., Hoekstra, R., Wehrens, R. and van Hintum, T. (2020). Effects of climate change on the distribution of crop wild relatives in the Netherlands in relation to conservation status and ecotope variation. Glob. Ecol. Conserv., 23, e01054. doi:https://doi.org/10.1016/j.gecco.2020.e01054
Vargas, J. H., Consiglio, T., Jørgensen, P. M. and Croat, T. B. (2004). Modelling distribution patterns in a species-rich plant genus, Anthurium (Araceae), in Ecuador. Divers. distrib., 10(3), 211-216. doi:https://doi.org/10.1111/j.1366-9516.2004.00081.x
Vignali, S., Barras, A. G., Arlettaz, R. and Braunisch, V. (2020). SDMtune: An R package to tune and evaluate species distribution models. Ecol. Evol., 10(20), 11488-11506. doi:https://doi.org/10.1002/ece3.6786
Warren, D. L., Glor, R. E. and Turelli, M. (2010). ENMTools: a toolbox for comparative studies of environmental niche models. Ecography, 33(3), 607-611. doi:https://doi.org/10.1111/j.1600-0587.2009.06142.x
Williams, J. W. and Jackson, S. T. (2007). Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ., 5(9), 475-482. doi:https://doi.org/10.1890/070037
Wisz, M. S., Hijmans, R. J., Li, J., Peterson, A. T., Graham, C. H., Guisan, A. and NCEAS Predicting Species Distributions Working Group. (2008). Effects of sample size on the performance of species distribution models. Divers Distrib., 14(5), 763-773. https://doi.org/10.1111/j.1472-4642.2008.00482.x
Yackulic, C. B., Chandler, R., Zipkin, E. F., Royle, J. A., Nichols, J. D., Campbell Grant, E. H. and Veran, S. (2013). Presence-only modelling using MAXENT: when can we trust the inferences? Methods Ecol. Evol., 4(3), 236-243. doi:https://doi.org/10.1111/2041-210x.12004
Zhang, L., Liu, S., Sun, P., Wang, T., Wang, G., Zhang, X., and Wang, L. (2015). Consensus forecasting of species distributions: the effects of niche model performance and niche properties. PloS one, 10(3), e0120056. https://doi.org/10.1371/journal.pone.0120056